New studies show similar-looking proteins behave very differently
Somdatta Karak
Computational tools like AlphaFold, whose developers won the chemistry Nobel Prize in 2024, predict the 3D structures of proteins in living cells. These data help scientists estimate which part of a protein will bind to which molecule and thus what the protein does. The more proteins scientists understand with precision, the more equipped they are to engineer proteins to have the desired outcomes.
But as scientists are studying proteins in greater detail, they are also uncovering peculiar new complications that existing structure-determining tools don’t anticipate.
For example, two studies from the CSIR-Centre for Cellular and Molecular Biology, Hyderabad, have found that two very similar proteins actually function very differently. The study from Rajan Sankaranarayanan’s lab shows that such details can be hidden in a building block of a protein that’s actually located far from the site at which the protein binds to a cell. The other from Mandar Deshmukh’s lab suggests the function also depends on how flexible a protein is.
An unexpected spot
Scientists look at the properties of protein where molecules bind. But the Sankaranarayanan lab’s work, in Nature Communications, urges us to zoom out.
The team focused on two proteins of the aminoacyl-tRNA synthetase (aaRS) group. These proteins help make other proteins in cells. Each aaRS protein picks a specific amino acid from 20 options and attaches it to specific tRNAs. The tRNAs offer the amino acids to a growing protein in a cell. A cell risks making dysfunctional or useless proteins if tRNAs bring the wrong amino acids. If the cell makes this mistake often, it will die.
aaRSs avoid such penalties using a proofreading mechanism. If an aaRS picks the wrong amino acid, its editing domain can detect the error and edit it. However, between two aaRSs with seemingly identical editing domains – AlaRS and ThrRS, the scientists found ThrRS was prone to adding the wrong amino acid to tRNA if the cell is stressed. The stressed cells also didn’t mind the mistake.
The scientists examined these proteins under powerful microscopes to understand why they behave differently when stressed. They found that the editing domain of AlaRS, the more correct aaRS had a zinc ion bound to it. This binding kept its editing mechanism intact in the stress condition. On the other hand, the editing domain of ThrRS didn’t hold zinc in the same pocket and thus its proofreading function went for a toss.
Looking at the protein region further away from the zinc-binding pocket, they found the two aaRSs differed by a single amino acid. The amino acid of the error-prone aaRS subtly altered the molecular architecture of the editing domain such that zinc didn’t prefer to bind at that spot.
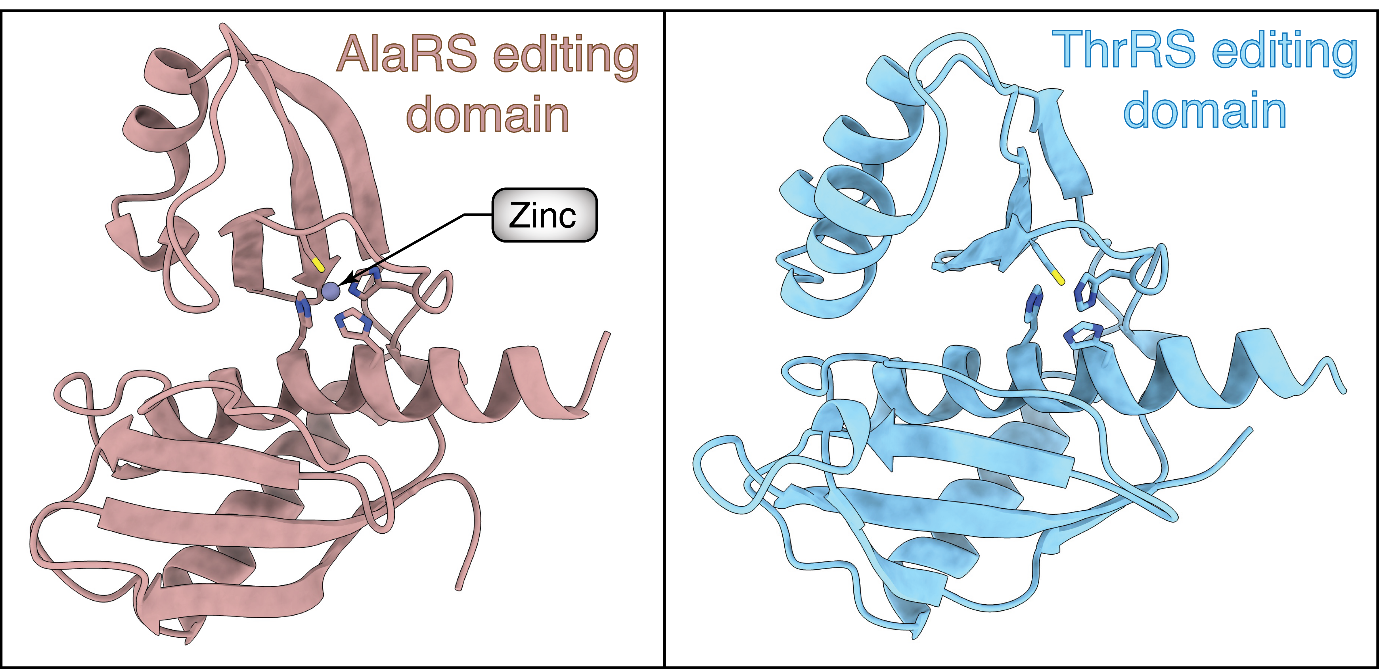
Structures of AlaRS and ThrRS editing domains
The team also found that the difference between the two aaRSs likely arose about 3.5 billion years ago, before bacteria and archaea diverged from their last universal common ancestor. The team suggested that the faltering ThrRS’ mistake was possibly used as a signal for the early cells to muster their overall protective mechanisms to counter environmental stresses, e.g. extreme temperature or high levels of ultraviolet radiation.
Because of the unique advantage the mutation conferred, what was originally a bug became a feature in the course of evolution.
Fleeting structures
Like cells, scientists also take advantage of slight differences in protein structures. They have designed drugs against cancer, viruses, allergies, ulcers, and mental health problems that block specific protein pockets.
Scientists identify these pockets in snapshots of protein structures. Many techniques capture images representative of proteins’ dominant and more stable shapes — but proteins don’t have fixed shapes. Their pockets can open and close, for example. And if these shape-changing events are short-lived, microscopy imaging techniques will likely miss them.
But Deshmukh’s group found a way to identify such fast-paced events in plant proteins that help deal with stress. Their paper, published in the Journal of American Chemical Society, detailed the dynamic nature of two similar proteins called DRB-2 and DRB-3.
The group used nuclear magnetic resonance, a technique that can detect if a specific chemical bond is more stretched in a molecule than others and fast enough to pick movements at even picosecond levels.
The scientists found that the pockets of DRB-2 and DRB-3 opened and closed differently. DRB-2 was a more rigid protein than DRB-3. DRB-3’s pockets, which help the plants detect stress, open and close at microsecond scales. This was long enough for other molecules to visit and dock.
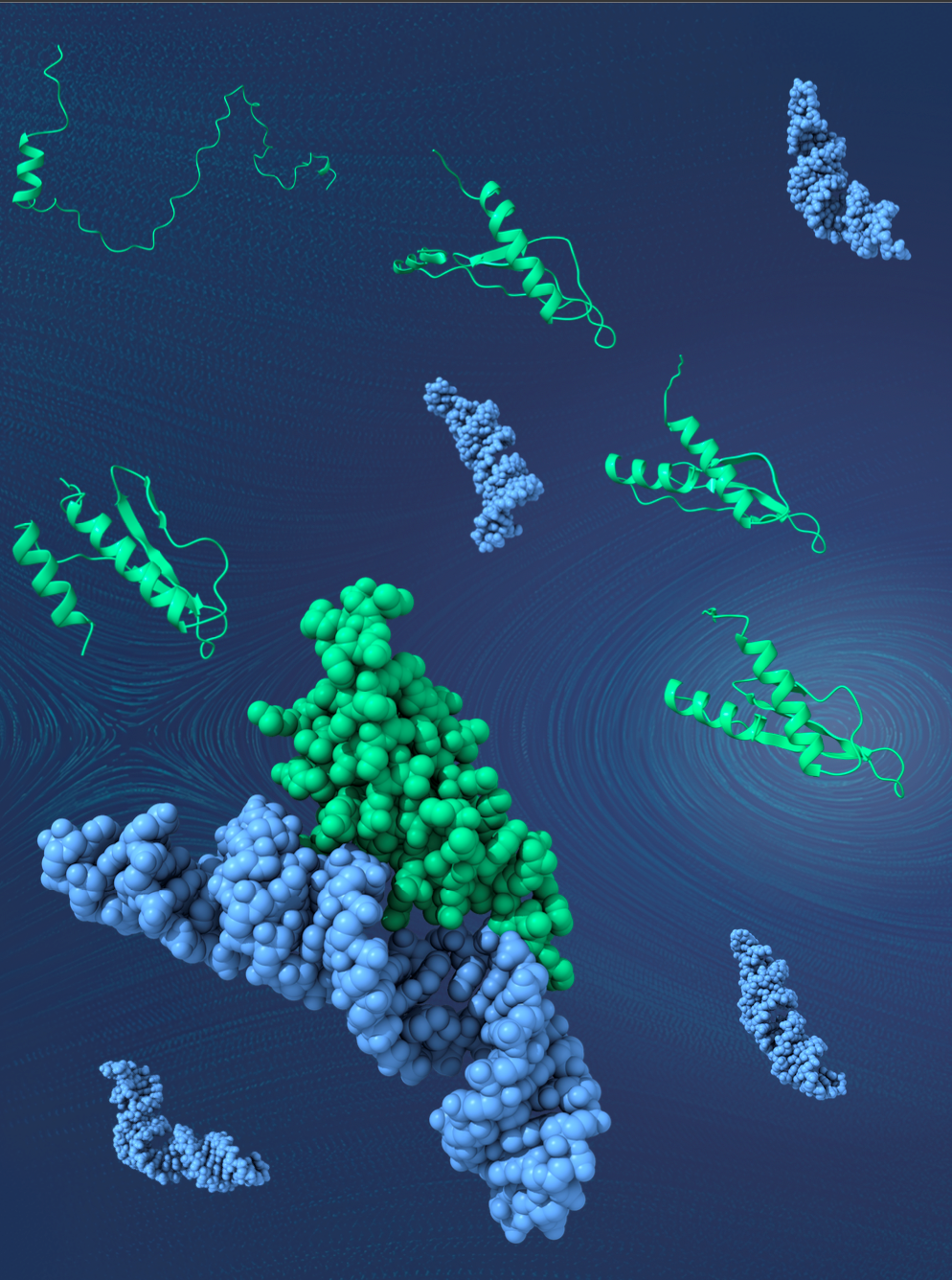
Artistic representation of the folding pathway of dsRNA binding proteins. It highlights the presence of folding intermediates that aid them in recognizing a diverse set of substrate dsRNA. PC: Mandar Deshmukh
Deshmukh also remarked that there were two different pockets on DRB-3 — one to detect environmental stresses such as salinity, flooding or droughts and another to sense infections.
When plants are less stressed, they fruit more. So, the scientists said, selective therapeutics can be designed to block the plants’ ability to detect environmental stresses by specifically targeting DRB-3.
Nuanced predictions
Thus, even though proteins’ overall 3D structures may look very similar, they can still operate very differently. For AlphaFold — which is among the current state-of-the-art — to go beyond the 3D structures of proteins and cover all protein functions, the teams said scientists will need fine details of protein structures and their dynamics.
These details currently don’t exist for most proteins. In fact, it’s very expensive to obtain them, up to crores of rupees per protein. The proteins often need to be extracted from their cellular environments before studying their high-resolution structures in procedures that demand painstaking customisations to suit each protein’s physical and chemical properties. Overall, there’s much trial and error involved in arriving at the methods to extract proteins while keeping their native conformations intact.
Yet it would seem in these little differences lie the details of life’s evolution, as well as hold clues to engineering novel therapeutics.
Somdatta Karak heads science communication at CSIR-CCMB.

 Pensioners Corner
Pensioners Corner Screen Reader Access
Screen Reader Access Skip to main content
Skip to main content






















