NMITLI
CSIR-New Millennium Indian Technology Leadership Initiative (NMITLI) Program
(Historical Journey from 2000-2019)
Background
Before the advent of globalization and liberalization era, the nation was more focused on self-reliance and indigenous development, which also guided the country’s objectives for technology development. High tariff barriers, limited competition in the domestic markets, poor integration with global markets, less emphasis on exports, greater focus on conserving foreign exchange rather than creating it, all led to an environment where S&T interventions were inward looking and determined by goals such as import substitution, conserving scarce foreign exchange and manufacturing without consideration for cost & level of technology. This resulted in country focusing largely on research areas where knowledgebase was already available and market demand existed. Whilst this approach suited well to the strategic sector(s), where technologies were not available at any cost or denied to us, this did not serve well the ends of competitive technology development. Public R&D institutions largely pursued the objectives of reverse engineering with focus on cost effective processes rather than novel products.
The industrial development model pursued by India, till the late eighties did not effectively encourage original R&D endeavor by industry. As a result, practically not a single technology originated / emerged from India which was globally competitive. The shift in the perception towards R&D began to change with the economic reforms of 1991 and a paradigm shift took place with India’s accession to WTO in 1995. However, accessing globally competitive industrial technology became difficult due to integration of the global trade / economy. Indian industry thus had no option but to develop such technology indigenously to survive. Several schemes for promoting industry-institute partnership for specific technology development initiatives then existed such as PATSER, Homegrown Technology etc. and a new scheme on promoting R&D in pharma industry was evolved in 1994 to address some of the concerns. However, there was none aimed at capturing global leadership position.
Genesis
The genesis of NMITLI was the address of the Hon’ble Prime Minister to the Indian Science Congress, at Pune on 3rd January 2000, wherein he called upon the scientific community to make the 21st Century India’s Century – Ikkeesvin shatabdi Bharat Ke Shatabdi. Dr. R.A. Mashelkar, the then Director General of CSIR submitted a conceptual note to Finance Ministry on February 17, 2000. Thereafter, the Hon’ble Minister for Science & Technology had enlarged upon the theme by exhorting the scientific community (on 21st February, 2000) to take up the challenge to create Indian Science that will lead and not follow.
The Hon’ble Finance Minister followed it up with a determined action. In his Budget - 2000 address to Parliament, he said that: “We must harness our potential in Science and Technology to realize the dream of modern India envisioned by the Prime Minister in his address to the Indian Science Congress last month……I am making a provision of Rs. 50 crore in the budget of the Department of Scientific and Industrial Research for launching a New Millennium Indian Technology Leadership Initiative. It will focus on areas which fulfill national objectives and will be based on partnership between the Government and private sector”.
The responsibility of conceptualizing, evolving and implementing the programme was assigned to the Council of Scientific & Industrial Research (CSIR).
Initiation
CSIR undertook national consultations in evolving the program. These included over 1000 letters from DG, CSIR to various opinion makers from all walks of life, over 65 brain storming meetings in various parts of the country, distribution of about 10,000 posters etc. Based on the responses received and discussions held, CSIR defined the main objective of the NMITLI as “to catalyze innovation centered scientific and technological developments as a vehicle to attain for Indian industry a global leadership position, in selected niche areas in a true ‘Team India’ spirit, by synergizing the best competencies of publicly funded R&D institutions, academia and private industry” and positioned it distinctively with novel features.
Novel Features
The strategy adopted for NMITLI is to obtain an inverse risk-investment profile i.e. low investment - high-risk technology areas (with global leadership potential) with investments increasing as developments take place and the projects move up on the innovation curve with reduction in risks. Therefore, the program has been positioned differently with certain distinctive features. These features have been evolved based on large scale national consultation and due diligence. Some of these are briefly highlighted below:
- A proactive program - Instead of funding a project based on requests/applications, the program identifies the areas for development based on national consultation and invites best partners from institutions, academia and private sector to play a role in the development;
- Types of Projects: Both ‘push’ and ‘pull’ type of projects are evolved under NMITLI, which are appropriately named as (i) Nationally Evolved Projects (NEP) and (ii) Industry Originated Projects (IOP);
- PPP mode - Almost all projects are built in a public-private partnership mode;
- Emphasis on identifying and building the projects - Greater emphasis is laid on identifying the niche areas and building the projects with the help of best brains in the country. A specially constituted project wise expert group builds the project by interacting with a large number of researchers and stake holders with focus on technology development;
- S&T inputs - High quality technical inputs are provided at both project development as well as at implementation stage;
- Monitoring & review system - A two-tier tight monitoring system is introduced to ensure realization of the objectives and deliverables. At the first level is an internal Steering Committee comprising PIs (meets once in 3 months) and at the second level an external independent Monitoring Committee comprising recognized peers (meets at least once in six months). The later committee is entrusted with the responsibilities to recommend: (i) foreclosure or modification of the project or sub component; (ii) inclusion of additional institutional / industrial partners wherever necessary; and (iii) revising the funding support to any / or all implementing partners;
- IP mapping - The program provides for continuous mapping of the IP scenario for each project and in licensing of IP with a view to building of a portfolio and achieving the leadership position;
- Foreclosure of projects - the program also provides for foreclosure of the non-performing or non-achievable project components; and
- Financial support – An innovative feature of the program is that it provides financial support to all players in the project. The support is in the form of grant-in-aid to the institutional partners in public domain and as a soft loan (@3% interest) to the industrial partners.
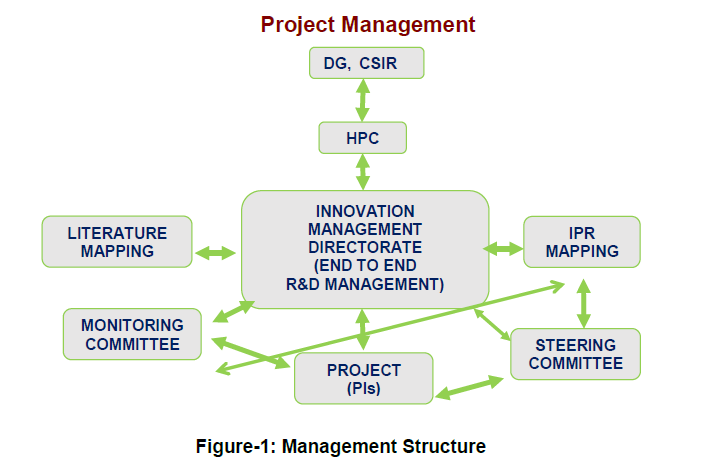
Management Structure
A dynamic and vibrant management system has been put in place to manage the Program and projects. At the hub of the management structure is the Mission Directorate, which manages the entire program. It interacts with PIs and the Monitoring Committee on one hand and the High Powered Committee, DG, CSIR and Governing Body of CSIR on the other.
Operational changes introduced
While approving the NMITLI scheme in March 2003, the CCEA had authorized the Governing Body of CSIR to effect operational changes (in the operation of the scheme) based on the exigencies of functioning in a dynamic global setting, within the overall financial outlays. Accordingly, the Governing Body of CSIR has introduced operational changes in the operation of the scheme which were felt necessary. These include: (i) Innovative capacity as criteria for NMITLI support to start-up companies; (ii) Rescheduling of loan repayment in select areas; and (iii) Financial support in the intermittent period. Brief details of these are presented below:
1. Innovative capacity as criteria for NMITLI support to startup companies
In case NMITLI restricts itself to consider only (as it does presently) the financial soundness of the company for extending financial support, it would end up supporting those set ups who are already on a strong financial footing. This situation is contrary to scenario world over and would deprive the startup companies bubbling with new and novel ideas, with out-of-box thinking and approach. Further, the risk taking capacities of these companies are much higher and they are eager to embark on newer frontiers in the realm of technology and business. Taking a liberal view on this issue in the light of cherished objectives of NMITLI program, GB, CSIR has decided to support such companies whose innovation capacity are high, utilizing up to 25% of NMITLI funds. To mentor such companies, in addition to existing monitoring mechanism, NMITLI would involve a financial institution like ICICI and one or two Technical Experts for necessary hand holding and regular guidance.
2. Rescheduling of loan repayment in select areas
The financial support under NMITLI is in the form of grant-in-aid to the institutional partners in public domain and as soft loan with 3% interest to the private sector industrial partners. The repayment of the loan component as well as interest by the industry partners is in 10 yearly installments which commences within six months of the completion of the project or within 18 months of release of last installment of loan whichever is earlier. This procedure is being followed where loan repayment has become due.
While operating the NMITLI Scheme for last few years, some difficulties in the procedure followed have been experienced. These are as follows:
- Depending upon the progress of the project, if additional work is required to be undertaken in order to fine tune / validate the technology product concept, extension of the project is provided based on Monitoring Committee recommendations. In such cases a situation arises where industrial partner has to pay back loan installments while the project is still under execution. It needs to be corrected, and a provision is required to be introduced so that loan repayment starts only after completion of the project(s).
- Some projects in the scheme fall under the area of Drugs & Pharmaceuticals. The projects normally start either at ‘Proof of Concept’ stage or at the stage of pre-clinical research. Leads obtained from the above projects have to essentially go through Investigational New Drugs (IND) application and regulatory process (Phase-I, II & III clinical trials in human subjects) before becoming a commercializable product. In the present situation, loan given to industry for projects at the stage of proof of concept/pre-clinical has to be returned while industry is conducting clinical trials on the formulation. Thus, industry needs to invest on two fronts i.e. for clinical trials and for loan repayment. Many a times, NMITLI funds these projects for conducting clinical trials (phase-II projects) also. This creates a situation where on one hand industry is receiving funds for clinical trials and on the other refunding the installment of previous loan.
3. Financial support in the intermittent period
The projects which move from ‘proof of concept’ stage to the next stage require development of a new project. Usually development of such projects and their approvals take considerable time. There was no provision for maintaining desired R&D activities during the intermittent period. Therefore, in order to maintain the pace of desired R&D activities and provide continuity, CSIR decided to provide financial support in the intermittent period to those projects which were recommended to move from one phase to other.
Implementation of NMITLI
Types of Projects
Keeping in view the objectives, NMITLI evolves both ‘push’ and ‘pull’ type of projects, which are appropriately named as (i) Nationally Evolved Projects (NEP); and (ii) Industry Originated Projects (IOP). The selection and development of projects follow a rigorous process with several stages of filtering through expert interventions. The best brains in the country are then entrusted with the responsibility of developing focused projects through intensive consultations and discussions with stake holders. The projects thus developed are considered and reviewed by a specially constituted High Powered Committee (HPC), comprising experts from diverse fields including financial institutions. The recommendation(s) of HPC are finally considered by CSIR Governing Body for necessary approval or otherwise of the project(s).
Flow diagrams for Nationally Evolved Projects (NEP) and Industry Originated Projects (IOP) are given below:
Stringent Selection Process
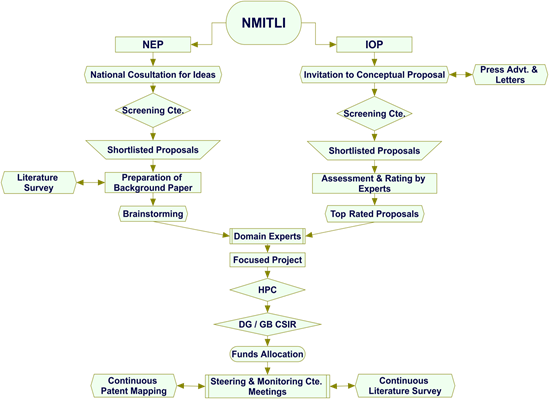
The Step-wise procedure for both Nationally Evolved Projects (NEP) and Industry Originated Projects (IOP) are briefly given in Annexure-1.
Rigor of scrutiny
Following the step-wise procedures, IOP proposals are subjected to high rigor of scrutiny at different stages. The following table illustrates the rigor of scrutiny applied in case of conceptual proposals received from the industry:
| Year | No. of Proposals received | No. of Proposals short listed by Screening Committee | No. of Proposals short listed by Area wise Experts for development | IOP Projects developed / launched |
|---|---|---|---|---|
| 2002 | 60 | 10 | 3 | 3 |
| 2003 | 163 | 24 | 5 | 3 |
| 2004 | 95 | 12 | 3 | 3 |
| 2005 | 90 | 21 | 4 | 1 |
| 2006 | 120 | 23 | 7 | 4 |
| 2007 | 100 | 29 | 8 | 7 |
| 2008 | 101 | 17 | 5 | 2 |
| 2009 | 130 | 24 | 11 | 6 |
| 2010 | 69 | 27 | 16 | 7 |
| 2011 | 57 | 23 | 7 | 1 |
| 2012 | 76 | 12 | 6 | 1 |
| 2013 | 70 | 21 | 12 | 4 |
| 2014 | 64 | 18 | 9 | 9 |
| 2015 | 59 | 25 | 8 | 5 |
Projects developed
Till 2019, NMITLI has implemented 79 largely networked projects in diverse areas viz. Agriculture, Healthcare, engineering, Energy, Chemicals and Information and Communication Technology. These projects involved 99 industry partners & 318 R&D groups from different institutions. Approximately 1700 researchers were engaged in these projects.
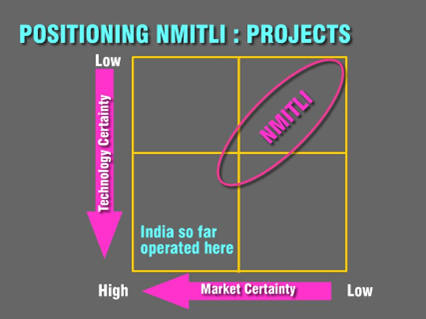
Positioning of NMITLI
The positioning of the projects is shown in Figure below. The constant endeavor of the program is to position the projects in the upper right most quadrant where the technology and markets are less known. Consequently, the risks and rewards associated with the projects are very high. Thus the strategy adopted for NMITLI was to obtain an inverse risk-investment profile, i.e. low investment – high risk technology areas (with global leadership potential) with investments increasing as developments take place and the projects move up on the innovation curve with reduction in risks.
Monitoring and Review
For closely monitoring and reviewing the projects, a two tier system is adopted which ensures realization of the objectives and deliverables. At the first level, there is an internal Steering Committee comprising Principal Investigators (PIs), which meets once in 3 months, monitors and sets its own periodic targets to be achieved. At the second level, an external independent Monitoring Committee comprising recognized peers reviews the project once in six months and guides the project towards its final objectives. Besides reviewing the progress of the project in conformance with the objectives, the later committee is entrusted with the responsibilities to recommend: (i) foreclosure or modification of the project or sub component; (ii) inclusion of additional institutional / industrial partners wherever necessary; and (iii) revising the funding support to any / or all implementing partners.
Expanding the activities of NMITLI
As India is entering into a new era of R&D activities and innovation led development, new approaches which spur innovation and further development need to be experimented under NMITLI. Accordingly, it is proposed to expand the NMITLI program both horizontally and vertically to experiment newer ways of conducting R&D for innovation. These new approaches are described below:
1. Funding along with industry (50:50 initiatives)
There are many Indian companies who are doing financially very well but do not have the necessary expertise and intellectual resources to develop focused network projects for development of technologies/products in their line of activities. Their efforts need complementation from suitable R&D institutions and guidance from recognized peers to develop and commercialize newer technologies/products. Therefore, NMITLI proposes to leverage its experiential base to encourage and assist such companies for developing network projects for those companies in product/technology development through a specific scheme, proposed to be called as ’NMITLI 50:50 initiatives’. The projects will be developed and implemented with same rigor as that of other NMITLI projects. This initiative would be made applicable to all Indian or foreign companies having manufacturing base in India. It is envisaged to provide 50% financial support from NMITLI as grant and the rest 50% project cost would come from the company for development and commercialization of the technology/product. On successful commercialization of the technology/product, the company shall pay a commensurating royalty to CSIR.
2. Co-financing with Venture Capital Funds
Many venture capitals are limited in scope and risk taking, due to lack of domain knowledge within the organization. Venture Capitals are therefore interested in joining hands with NMITLI, which has strong domain knowledge base, to jointly finance projects. Such projects would be identified and evolved following the procedures established by NMITLI. The funding would be joint with pre-determined ratio, but not more than 50% contribution from NMITLI. These projects are envisaged to be monitored by a joint team of experts as per the NMITLI monitoring mechanism. The proposed funding would follow the venture funding norms. The successes and failures resulting from the projects will be shared on equitable basis. This initiative would encourage venture capital funds to venture into riskier R&D areas.
3. Joint development and support of projects with other departments of science and technology and economic ministries
Many government departments particularly in economic ministries are engaged in research and development activities in areas of relevance to them. These activities often have considerable degree of overlap with other scientific departments. However, these departments’ expertise is limited to undertake multi-disciplinary projects in cutting edge areas requiring wide-spectrum of intellectual and infrastructural inputs. Such multi-disciplinary areas need expertise, inputs and concerted efforts from all concerned government departments to generate IPR, technologies and products besides high quality publications. Therefore, it is proposed to utilize part of the NMITLI funds to generate inter-departmental projects in the XI FY Plan. The proposed scheme apart from generating intellectual capital, technologies and products in cutting edge areas would act as a catalyst to bring better co-ordination among various departments of government in the R&D sphere.
4. Setting up of NMITLI Innovation Centers in selected areas for long term sustained efforts
In many areas, country has limited expertise scattered across different institutions. Such diffused expertise has not led to any worthwhile innovation in those areas. Even the networking of NMITLI would not be very effective in such cases because these areas would require long term commitment and creation of facilities at different locations. Therefore, these areas need long term sustained support with requisite human resource as well as infrastructure, assembled at one place to cross the threshold of intellectual barrier in order to generate globally competitive technologies and products, IPR, and high quality publications. It is thus proposed to set up ‘NMITLI Innovation Centres’ in PPP mode for sustained efforts in some selected areas for example, Photovoltaics, Fuel Cells, White LEDs, Industrial Enzymes, Medical Implants, Vaccine development, Seed Development etc.
These centres would be set up in an identified institution or independently. These centres will work only in the identified area. To attract and retain best talent, the scientists engaged in the activity will receive Salary plus, where salary comes from government and the plus comes from industrial partners. As suggested by EFC, such centres will be created where it utmostly required.
The financial implications for setting up each such centre will be worked out and placed before GB, CSIR for approval.
5. Support to post NMITLI projects
Despite the excellent R&D and developments, the technologies and products developed in the laboratory do not reach to the market due to lack of ‘last mile approach’. The companies need CSIR’s hand holding to develop and package the technologies/products further and commercialized it. The concept of ‘Post-NMITLI’ is being proposed to fulfill the objective of providing financial and technical assistance for pre-commercialization related activities such as scale up, pilot plants, field trials, market seeding of products, market surveys, etc. The proposed assistance in case of industry, would be in the form of soft loan. Even these Post-NMITLI projects would be monitored on the same lines as that of NMITLI vis-à-vis pre-defined objectives.
6. Acquisition of early stage relevant knowledge / IP for portfolio building
External ideas / leads / IP acquisition are assuming greater significance in the chain of innovation and mind to market. The availability of a large number of unencumbered IP (being developed in several laboratories globally) is providing a fillip to this approach. Several countries across the globe are striving to take advantage of the diversity of creativity available in different parts of world and integrate with its own developments to bring out new products / processes for global competitiveness. Since NMITLI aims to provide the Technological Leadership to the Indian industry, it becomes imperative for NMITLI to adopt such practices to achieve its objectives. As recommended by the EFC, such acquisitions shall be limited to carefully chosen areas with a view to creating a portfolio where NMITLI projects are in operation. Before acquiring IP, necessary due diligence will be carried out and the proposal will be placed to competent authority for approval.
7. Crossing the boundaries
It is increasingly being felt that to achieve leadership in niche technology areas, relying totally on internal expertise and capabilities may not be adequate. To achieve the objective of global leadership, it would be helpful to broaden the program by bringing in international expertise. The international expertise may be in the form of (i) expert advice of international experts at various stages of project development and implementation; (ii) involving international companies for product/technology development and commercialization at global scale; and (iii) engaging research institutions and/or CROs across the globe where Indian expertise need outside complementation. Thus, NMITLI seeks to draw upon or outsource the expertise, wherever it is available at a global level. The proposal would inter-alia involve relaxing the condition of Indian institutions and companies could only be partners in the NMITLI as well as releasing funds in foreign currency for drawing upon experts and expertise from abroad. As recommended by the EFC, the provision will be used selectively.
While implementing the proposal, GFR provisions, to the extent possible and feasible shall be followed. Wherever, a change is required, approval of GB, CSIR shall be obtained.
While implementing the proposal, GFR provisions, to the extent possible and feasible shall be followed. Wherever, a change is required, approval of GB, CSIR shall be obtained.
Annexure-1
Step-wise procedure for developing the Nationally Evolved Projects (NEP) under NMITLI
I. National Consultations
- Wide-spread and wide-ranging national consultations are held to identify the potential niche ideas. The consultations are usually done through letters from DG, CSIR to eminent personalities in all walks of life.
- (ii) The suggestions so received are compiled into a database.
II. Selection of suggestions / ideas
The two-tier mechanism as given below:
- A Screening Committee comprising of experts including senior officers from Government departments with wide exposure over different disciplines shortlist the suggestions / ideas for their conformance to NMITLI objectives;
- The Screening Committee’s recommendations are placed to DG, CSIR for his consideration, review and confirmation; and
- The short listed suggestions / ideas are then categorized into different areas, e.g. Agriculture, Biotechnology, Chemicals, Drugs and Pharmaceuticals, Energy, Materials, Information and Communication Technology etc.
- Separate Expert Groups are constituted by DG, CSIR to consider the short listed suggestions / ideas for selecting those of the few ideas that could provide an advantage to India. Each of the Expert Group has 4-5 experts from the relevant areas;
- The selected suggestions / ideas are then put up to DG, CSIR for his consideration, review and approval; and
- Selected ideas are then communicated to all the contributors
In addition, CSIR proactively scout for project ideas through interaction with industry and researchers. This is done by organizing interactive meetings with researchers in specific areas to catalyze potential project ideas. The selected ideas are then taken through a brainstorming meeting and the concepts are placed before DG, CSIR for his consideration and approval. These concepts are then developed into projects following the subsequent steps.
III. Preparation of Project Proposals
- For each selected idea, a Specialist Expert Group (Champions Group) is commissioned to evolve the selected idea into a project. The Group comprises of three to four experts in the relevant area with at least one of them being from industry, to provide an industrial perspective to the project. The Champions Group is provided a free hand in zeroing in on the niche segment within the selected area, developing it into a project, selecting the (best) partners, project time frame and budget estimates etc.
- Towards evolving the project, the Champions Group, besides having internal meetings among themselves, have at least one meeting with the domain specific researchers from academia, institutions & industry.
It is important to recognize that participation in NEP category of NMITLI projects is not by application, but by invitation. Further, individuals who suggested an area may or may not be part of the research project
IV. Involvement of Industrial Partners
Some of the approaches among others that are followed to enlist the industry partners are:
- Industrial partners are invited for the brainstorming meeting at the time of evolving the projects;
- Where the industry participation in the brainstorming meeting is minimal or nil, CSIR specially addresses letters to the firms in that industry sector for soliciting their interest;
- Earlier TNBD and now Mission Directorate at CSIR independently also interacts with the relevant industries and solicit their participation in the project; and
- Firms that show interest in participating in the program are enlisted in the projects.
V. Approvals
- The final project as developed is submitted for consideration of a High-Powered Committee (HPC), which is constituted by DG, CSIR. In facilitating the HPC to arrive at a decision, the Champions are invited to make a presentation to the HPC; and
- The project proposal along with HPC comments / recommendations including the funding aspect is then put up to DG, CSIR / GB(SFC) / EFC as appropriate for final consideration and approval.
VI. Disbursal of Funds
A MOU / legal agreement is entered among the partners and funds are then released to implement the project
Step-wise procedure for developing Industry Originated Projects (IOP) under NMITLI
I. Soliciting conceptual ideas/proposals
- Through press advertisement in leading national newspapers inviting conceptual proposals from Indian industry under the scheme; and
- Letters from CSIR are addressed to a large number of Indian industries inviting conceptual ideas / proposals for support under the scheme.
II. Selection of conceptual proposals
- Compilation of the conceptual ideas / proposals received within the specified time; and
- Prima facie evaluation of the conceptual ideas / proposals for their conformance to NMITLI objectives by a committee of experts drawn from different fields (set up by DG, CSIR).
- Assessment of short listed conceptual ideas / proposals by a committee of eminent technical domain experts (minimum of 3 experts) rated on 1 to 10 scale for further consideration and examination; and
- Selection of top rated one or two ideas based on average rating with a minimum rating of 5) in each broad area
III. Development of conceptual ideas into projects
Development of selected idea / conceptual proposal into project proposal with the guidance of national experts and concerned industry partner. CSIR identifies and provides these experts for helping the industry to build the proposal.
IV. Approvals
- The final project proposal as developed is submitted to the High-Powered Committee for consideration and recommendation; and
- The project proposal along with HPC recommendations is then put up to DG, CSIR/SFC(GB)/EFC, as appropriate for final consideration and approval.
V. Disbursal of Funds
An agreement is entered among the partners and then funds are released to partners for implementation of the project.
Annexure-2
List of NMITLI projects (2000-2019):
| S.No. | Project Title |
|---|---|
| 1 | Two orders of magnitude improved liquid crystals for flat panel display devices |
| 2 | Defunctionalization of carbohydrates as a feed stock to manufacture well identified industrial chemicals |
| 3 | New targets and markers for cancer using genomics and proteomics |
| 4 | Stimuli sensitive polymeric nano-particle based advanced drug delivery systems for cancer, diabetes and anti-bacterials |
| 5 | 5 & 25 KW decentralized power packs |
| 6 | Latent M. Tuberculosis: New Targets, Drug Delivery system, bio-enhancers and therapeutics |
| 7 | Using Functional genomics in tea, mentha, Ashwagandha, plants for gene expression modulation |
| 8 | Meso-scale modeling for monsoon predictions-Phase II |
| 9 | Nano-material catalysts and associated process technology for alkylation/ acylation/nitration of well identified industrial chemicals, pre-reforming of hydro-carbons and sulphur removal (<50 ppm) from petroleum fuels |
| 10 | Versatile, portable PC based software for bioinformatics & Development of Linux cluster version of Bio-suite |
| 11 | Biodegradable plastics from agricultural wastes: Cellulose esters based on bagasse & Steam explosion-based fractionation process of sugarcane bagasse to cellulose, hemicellulose and lignin-Validation at semi-commercial level |
| 12 | Herbal based preparations for degenerative disorders: Diabetes mellitus type II, Osteoarthritis and Rheumatoid arthritis and Common hepatic disorders |
| 13 | Biotechnology for replacing chemical process in leather Sector-Phase-I & II |
| 14 | Enhanced productivity in cement manufacture through improved granular processing and resource conservation |
| 15 | Development of an Oral herbal formulation for treatment of Psoriasis a clinical and scientific challenge & Clinical studies of an oral herbal formulation for treatment of psoriasis & Phase-IIb & III Clinical Trials |
| 16 | Development of novel biotech therapeutic molecule –Lysostaphin & Phase-II Clinical studies: Novel bio-therapeutic molecule-Lysostaphin & Novel Biotherapeutic Molecule: Lysostaphin – Phase IIb/III Clinical studies |
| 17 | Microbiological conversion of Erythromycin to Clarithromycin and other novel biologically active molecules |
| 18 | Environmentally secure rare earth based colorants for surface coatings & Phase II |
| 19 | Functionalization of alkanes |
| 20 | Novel molecular diagnostics for eye diseases and low vision enhancement devices & Development of DNA Macroarray for the detection of eye infections and the genetic predisposition to glaucoma |
| 21 | Nano-material coatings and advanced composites for tribological applications in automotive industry |
| 22 | Value added polymeric materials from renewable resources: Lactic acid and lactic acid based polymers & Lactic acid and lactic acid based polymers-establishment of 300 TPA pilot plant for lactic acid production |
| 23 | Recombinant approach to produce a-linolenic acid and docosahexanoic acid (DHA) in sunflower and yeast |
| 24 | A cost effective Simple Office Computing (Sofcomp) platform to replace PC |
| 25 | A PC based high-end 3D visualization platform for computational biology – ‘Darshee’ |
| 26 | Improved Genome Annotation Through a Combination of Machine Learning and Experimental Methods: Plasmodium falciparum As a Case Study |
| 27 | Oral Delivery of Insulin |
| 28 | Pharmacological and Genomic Investigations on Withania somnifera - An Indian Medicinal Plant & Leads based drug development and genetic improvement of Ashwagandha (Withania somnifera) |
| 29 | Development of Novel Fungicides |
| 30 | Biotechnological Approaches for Improvement of Plant Species with Special Reference to Pulp and Paper |
| 31 | Development of fuel cells based on Hydrogen & Development and demonstration of polymer electrolyte fuel cell stacks for stationary applications & Demonstration and Validation of 3kWe PEMFC System |
| 32 | Development of a 500 kW low cost horizontal-axis Wind Turbine |
| 33 | Novel expression system |
| 34 | Development of selected Medical Implants And Development of Dental Implants-Phase II study |
| 35 | Genetic Improvement of Jatropha curcas for Adaptability and Oil Yield |
| 36 | Development of Globally competitive ‘Triple-Play’ Broadband Technology |
| 37 | Novel formulation for treatment of pulmonary tuberculosis – clinical studies |
| 38 | Market seeding of SofComp and Mobilis to develop wide-ranging applications as well as increase awareness |
| 39 | Process for Tamiflu – a Blockbuster drug to combat the menace of avian flu |
| 40 | Development of production system for Tea Polyphenols and their condensed products |
| 41 | Development of an integrated micro PCR system with In-situ Identification & Validation of handheld diagnostic platform for HBV detection |
| 42 | A prospective study to correlate gene signatures with clinical outcome of astrocytomas and identification of potential therapeutic target(s) |
| 43 | Development of high throughput marker assisted selection systems for improvement of drought tolerance and fibre quality related traits in cotton |
| 44 | Novel method for development of B-type Natriuretic peptide (BNP) for diagnosis and treatment of congestive heart failure |
| 45 | Development of Next Generation Plasma Display Technology and a 50 inch High Definition (HD) TV prototype |
| 46 | Wireless Sensor Network Chipset based on Ultra –Wideband Technology |
| 47 | Conversion of cellulose and hemi-cellulose into sugars and ethanol |
| 48 | Conversion of Bioglycerol into value added chemicals & Scale up of CSIR-NCL Process for selected hydrogenolysis of glycerol to 1, 2-Propan-di-ol |
| 49 | Novel approaches for production of hybrid seeds with characteristics of improved insect resistance and higher yield |
| 50 | Meso-scale modeling for Monsoon predictions - Phase II |
| 51 | Design and development of cushion bonded organic ceramic clutch discs |
| 52 | Intelligent video surveillance system |
| 53 | Novel therapy for management of sepsis and Novel therapy for management of sepsis Phase II study |
| 54 | A syndromic approach to diagnosis of infections: development of DNA macro-chips for simultaneous detection of pathogens causing AES (Acute Encephalitic Syndrome) and septicaemia |
| 55 | Evaluation of RNAi-based constructs for conferring resistance on transgenic rice against the blast fungus Magnaporthe grisea |
| 56 | Development and characterization of an indigenous vaccine for Johne’s disease |
| 57 | Development of Caerulomycins as novel immunosuppressive agents to prevent organ rejection after transplantation and to address various auto-immune and allergic disorders |
| 58 | Novel DPP IV inhibitors for the treatment of Diabetes & Novel DPP IV inhibitor – Phase I/II Study: A safety, pharmacokinetic and pharmacodynamics study of CPL-2009-0031 in Healthy Volunteers and patients with Type 2 Diabetes mellitus (T2DM) |
| 59 | Biofuel from marine microalgae |
| 60 | Development and production of a therapeutic monoclonal antibody against eNAMPT, a novel inflammatory target |
| 61 | Biomarker Neck and Head Cancer |
| 62 | Design, Development and demonstration of high performance parabolic trough based 300 kW Solar Thermal Power Plant |
| 63 | Development and demonstration of 500 W SOFC Stack with hydrogen as fuel and testing of short stack with synthetic gas & Development and demonstration of 1 kWe SOFC Stack with hydrogen & Air and 500 We SOFC Stack with reformed natural Gas and Air |
| 64 | The next generation wind power technology: contra-Rotor Wind Turbine (CRWT) System |
| 65 | Development and commercialistion of “Vennfer” – unique H.264 high definition software based multiparty, multipoint video conferencing solution on multipoint network transmission protocol |
| 66 | Development and commercialization of NXR-4D: A lithium-Ion battery powered 4-door next generation electric Car (4-Seater Capacity) - Mahindera Reva Electric Vehicles Pvt. Ltd., Bangalure |
| 67 | Design and fabrication of All-Fibre Supercontinuum Light Source with application demonstration to detect fake pills: Vinvish Technologies Pvt. Ltd., Thiruv’puram |
| 68 | Development and commercialization of Soleckshaw Lite – An Innovative Electrical Green Transport Platform: Kinetic Engineering Ltd., Pune |
| 69 | Customized adaptation of nonClonableID technology to establish authenticity of medical products: Bilcare Ltd., Pune |
| 70 | Development of a diagnostic system for affordable, point of need testing to manage HIV and TB: Reametrix India Pvt. Ltd., Bangaluru |
| 71 | Development of non-hazardous process for the synthesis of Di Methyl Carbonate (DMC) from methanol and urea |
| 72 | System based computational model of skin (SCoMOS): CSIR-IGIB, Delhi |
| 73 | Integrated technological solutions for security and operations based on UV sensor technologies |
| 74 | Design and development of photonic crystal cladded and double called Er and Er/Yb fibers, and application demonstration of high-power optical amplifier |
| 75 | Demonstration and validation of a 5 kW HT-PEMFC based combined cooling and power system |
| 76 | Demonstration and Validation of a LT-PEMFC system for automotive application |
| 77 | Development of an antidiabetic agent based on the phytopharmaceutical drug guidelines from enicostemma littorale blume |
| 78 | Kappaphycus alvarezii and red seaweed based formulations for improving productivity and health of dairy and poultry animals |
| 79 | Automation of Ghani operation through vacuum conveying system |
Annexure-3
NMITLI Projects at a Glance (Since the start of Scheme)
Project under different domains
| Healthcare | Agriculture | Engineering |
|---|---|---|
1. New targets and markers for cancer using genomics and proteomics 2. Stimuli sensitive polymeric nano-particle based advanced drug delivery systems for cancer, diabetes and anti-bacterials 3. Latent M. Tuberculosis: New Targets, Drug Delivery system, bio-enhancers and therapeutics & Clinical Trials 4. Herbal based preparations for degenerative disorders: Diabetes mellitus type II (NIDDM) with emphasis on insulin sensitization and Herbopoint & Herbal based preparations for degenerative disorders: Osteoarthritis and rheumatoid arthritis especially for pain management & Herbal based preparations for degenerative disorders: Common hepatic disorders with emphasis on hepatocellular protection and herboprint 5. Development of an Oral herbal formulation for treatment of Psoriasis a clinical and scientific challenge 6. Development of novel biotech therapeutic molecule –Lysostaphin & Novel Biotherapeutic Molecule: Lysostaphin – Phase IIb/III Clinical studies 7. Microbiological conversion of Erythromycin to Clarithromycin and other novel biologically active molecules 8. Novel Molecular Diagnostics for Eye Diseases and Low Vision Enhancement Devices & Development of DNA Macroarray for the detection of eye infections and the genetic predisposition to glaucoma 9. Oral Delivery of Insulin 10. Process for Tamiflu – a Blockbuster drug to combat the menace of avian flu 11. A prospective study to correlate gene signatures with clinical outcome of astrocytomas and identification of potential therapeutic target(s). 12. Development of an integrated micro PCR system with In-situ Identification - Phase II 13. Novel method for development of B-type Natriuretic peptide (BNP) for diagnosis and treatment of congestive heart failure 14. Novel formulation for treatment of pulmonary tuberculosis – clinical studies 15. Novel therapy for management of sepsis 16. A syndromic approach to diagnosis of infections: development of DNA macro-chips for simultaneous detection of pathogens causing AES (Acute Encephalitic Syndrome) and septicemia 17. Development and characterization of an indigenous vaccine for Johne’s disease 18. Development of Caerulomycins as novel immunosuppressive agents to prevent organ rejection after transplantation and to address various auto-immune and allergic disorders 19. Novel DPP IV inhibitors for the treatment of Diabetes & Novel DPP IV inhibitor – Phase I/II Study: A safety, pharmacokinetic and pharmacodynamics study of CPL-2009-0031 in Healthy Volunteers and patients with Type 2 Diabetes mellitus 20. Development and production of a therapeutic monoclonal antibody against eNAMPT, a novel inflammatory target 21. Discovery and Validation of New Biomarkers for Head & Neck Cancer in India 22. Customized adaptation of non ClonableID technology to establish authenticity of medical products 23. Development of a diagnostic system for affordable, point of need testing to manage HIV and TB 24. Development of an antidiabetic agent based on the Phytopharmaceutical drug guidelines from Enicostemma littorale blume. 25. Development of selected Medical Implants & Development of Dental Implants-Phase II study | 1. Using Functional genomics in tea, Mentha, Ashwagandha, plants for gene expression modulation 2. Biodegradable plastics from agricultural wastes: Cellulose esters based on bagasse 3. Pharmacological and Genomic Investigations on Withania somnifera - An Indian Medicinal Plant & Leads based drug development and genetic improvement of Ashwagandha (Withania somnifera) 4. Novel expression system 5. Development of Novel Fungicides 6. Biotechnological Approaches for Improvement of Plant Species with Special Reference to Pulp and Paper 7. Genetic Improvement of Jatropha curcas for Adaptability and Oil Yield 8. Development of production system for Tea Polyphenols and their condensed products 9. Development of high throughput marker assisted selection systems for improvement of drought tolerance and fibre quality related traits in cotton 10. Novel approaches for production of hybrid seeds with characteristics of improved insect resistance and higher yield 11. Evaluation of RNAi-based constructs for conferring resistance on transgenic rice against the blast fungus Magnaporthe grisea 12. Kappaphycus alvarezii and Red seaweed based formulations for improving productivity and health of dairy and poultry animals. | 1. Meso-scale modeling for monsoon predictions and Mesoscale modeling for Monsoon Predictions-Phase II 2. Environmentally secure rare earth based colorants for surface coatings 3. Nano-Material Coatings and Advanced Composites for Tribological Applications in Automotive Industry 4. Development of Next Generation Plasma Display Technology and a 50-Inch-High Definition (HD) TV prototype 5. Design and development of cushion bonded organic ceramic clutch discs 6. Development and commercialization of NXR-4D: A lithium-Ion battery powered 4-door next generation electric Car (4-Seater Capacity) - 7. Design and fabrication of All-Fiber Supercontinuum Light Source with application demonstration on spectroscopic signature detection 8. Development and commercialization of Soleckshaw Lite – An Innovative Electrical Green Transport Platform: 9. Development of integrated technological solutions for security and operations based on UV sensor technologies 10. Design and development of photonic crystal cladded and double called Er and Er/Yb fibers, and application demonstration of high-power optical amplifier 11. Automation of Ghani operation through vacuum conveying system |
| 25 | 12 | 11 |
| Energy | Chemicals | Information Technology |
|---|---|---|
1. 5 & 25 KW decentralized power packs 2. Improved granular processing: towards energy efficiency and resource conservation in cement manufacture 3. Functionalization of alkanes 4. Development of fuel cells based on Hydrogen; Development and demonstration of polymer electrolyte fuel cell stacks for stationary applications; & Demonstration and Validation of 3kWe PEMFC System 5. Development of a 500 kW low cost horizontal-axis Wind Turbine 6. Biofuel from Marine Microalgae 7. Design, Dev. and demonstration of high performance Parabolic Trough based 300 kW Solar Thermal Power Plant 8. Dev. and demonstration of 500W SOFC Stack with hydrogen as fuel and testing of short stack with synthetic gas & Development and demonstration of 1 kWe SOFC Stack with hydrogen & Air and 500 We SOFC 9. The next generation wind power technology: contra-Rotor Wind Turbine (CRWT) System 10. Demonstration and validation of a 5KW HT-PEMFC based combined cooling and power system 11. Demonstration and validation of a LT-PEMFC system for Automotive application 12. Stack with reformed natural Gas and Air | 1. Two orders of magnitude improved liquid crystals for flat panel display devices 2. Defunctionalization of carbohydrates as a feed stock to manufacture well identified industrial chemicals 3. Nano-material catalysts and associated process technology for alkylation/ acylation/nitration of well identified industrial chemicals, pre-reforming of hydro-carbons and sulphur removal (<50 ppm) from petroleum fuels 4. Biotechnology for replacing chemical process in leather sector- Phase I & Phase II 5. Value added polymeric materials from renewable resources: Lactic acid and lactic acid based polymer & Lactic acid and lactic acid based polymers-establishment of 300 TPA pilot plant for lactic acid production 6. Recombinant approach to produce a-linolenic acid and docosahexanoic acid (DHA) in sunflower and yeast 7. Conversion of cellulose and hemi-cellulose into sugars and ethanol 8. Conversion of Bioglycerol into value added chemicals & Scale up of CSIR-NCL process for selective hydrogenolysis of glycerol to 1, 2- Propanediol- Phase 2 9. Development of non-hazardous process for the synthesis of Di Methyl Carbonate (DMC) from methanol and urea | 1. Versatile, portable PC based software for bioinformatics and Development of Linux cluster version of Bio-suite 2. A cost effective Simple Office Computing (Sofcomp) platform to replace PC 3. Market seeding of SofComp and Mobilis to develop wide-ranging applications as well as increase awareness 4. A PC based high-end 3D visualization platform for computational biology – ‘Darshee’ 5. Improved Genome Annotation Through A Combination Of Machine Learning And Experimental Methods: Plasmodium Falciparum As A Case Study 6. Development of Globally competitive ‘Triple-Play’ Broadband Technology 7. Wireless Sensor Network Chipset based on Ultra –Wideband Technology 8. Development of Intelligent video surveillance server system 9. Development and commercialization of “Vennfer” – unique H.264 high definition software based multiparty, multipoint video conferencing solution on multipoint network transmission protocol 10. System based computational model of skin (SCoMOS) |
| 12 | 9 | 10 |
Annexure-4
NMITLI projects (Nationally Evolved Projects)
| Year | Project Title | Institutional Partners | Industry Partners | Budget (Rs in lakh) |
|---|---|---|---|---|
| 2000-2003 | 1. Two orders of magnitude improved liquid crystals for flat panel display devices | CLCR, Bangalore | BEL, Bangalore | 147.000 |
| 2. Defunctionalization of carbohydrates as a feed stock to manufacture well identified industrial chemicals | NCL; RRL, Jammu; IICT; RRL, Triv.; IIT-M; UDCT, Mumbai; UDSC, New Delhi; TERI, New Delhi; ARI, Pune | GNFC, Bharuch; Prathista Ind. Ltd., Secunderabad & Anil Starch Product, New Delhi | 253.710 | |
| 3. New targets and markers for cancer using genomics and proteomics | CCMB; IISc.;TMC, Mumbai; RGCIRC, ND; Manipal, Bangalore; IACS, Kolkata | DRF, Ghaziabad | 1533.596 | |
| 4. Stimuli sensitive polymeric nano-particle based advanced drug delivery systems for cancer, diabetes and anti-bacterials | NCL; CDRI; DU, Delhi; DUSC, New Delhi; SCTIMST; UDCT; NIPER | Lupin Mumbai; Ranbaxy; Wockhart; USV & SUN Pharma | 315.532 | |
| 5. 5 & 25 KW decentralized power packs | NCL; SSF, Chennai | BHEL; Sud-Chemie, New Delhi | 609.740 | |
| 6. Latent M. Tuberculosis: New Targets, Drug Delivery system, bio-enhancers and therapeutics & Clinical Trials | NCL; IICT; CDRI; ITRC; IGIB; RRL, Jam.; UOH, Hyd.; NIPER; ICGEB; Bose Inst., Kolkata; IISc.; NII; CDFD | Lupin Ind., Mumbai | 3549.585 | |
| 7. Using Functional genomics in tea, mentha, ashwagandha, plants for gene expression modulation | NBRI; CIMAP; IHBT; RRL, Jam.; UDSC, New Delhi; MSU, Baroda; TRA, Calcutta; UPASI, Coimbatore | Essential Oil Association of India, New Delhi | 856.519 | |
| 8. Meso-scale modeling for monsoon predictions | NAL; C-MMACS; IISc.; IIT-D, Delhi; IITM, Pune; CU, Kochi; TIFR, Bengaluru | Encore Software, Bangalore; IWSTPL, Bang. | 815.057 | |
| 9. Nano-material catalysts and associated process technology for alkylation/ acylation/nitration of well identified industrial chemicals, pre-reforming of hydro-carbons and sulphur removal (<50 ppm) from petroleum fuels | NCL; IICT; CSMCRI; CFRI; RRL, Triv.; UICT; CPCL; HOCL | Sud-chemie, N.D. | 551.500 | |
| 10. Versatile, portable PC based software for bioinformatics and Development of Linux cluster version of Bio-suite | IMT; IICB; CDRI; IGIB; NAL; IIT-B; IIT-D; IIT-Khagpur; MKU; BI, Kolkata; NIPER; UOM, Chennai; IISc.; UOP, Pune; CDFD; IBAB; Saha, Kolkata; ISI, Kolkata; IIIT, Hyderabad | TCS Hyderabad; Jalaja, Bangalore; FITL, Hyderabad | 1628.320 | |
| 11. Biodegradable plastics from agricultural wastes: Cellulose esters based on bagasse | NCL; CPPRI; TCIRD; PPPML, Pune | EID Parry, Chennai; Godawari, Mumbai | 300.130 | |
| 12. Herbal based preparations for degenerative disorders: Diabetes mellitus type II (NIDDM) with emphasis on insulin sensitization and Herboprint | NBRI; IICB; IICT; RRL, Jam.; SPARC, Mumbai; TNMC, Mumbai; VKAN, Mumbai; NIMS, Hyd.; MDRF, Chennai; VSGH, Ahmedabad | ZPWL, Mumbai; AVS, Kottakal; AVP, Coimbatore; SDL, Mumbai; NRPL, Bang. | 417.535 | |
| Herbal based preparations for degenerative disorders: Osteoarthritis and rheumatoid arthritis especially for pain management | NBRI; IICT; RRL, Jam.; UOP-ISHS, Pune; BVDU-IRSHA, Pune; KEM, Mumbai; CRD, Pune; SPARC, Mumbai; NIMS, Hyderabad; AIIMS, New Delhi | ZPWL, Mumbai; DRF, Ghaziabad; AVS, Kottakkal; AVP, Coimbatore | 469.000 | |
| Herbal based preparations for degenerative disorders: Common hepatic disorders with emphasis on hepatocellular protection and herboprint | RRL, Jam.; NBRI; IICT; SPARC, Mumbai; PGI; CLRD-DMRC, Hyd.; SGSM&KEM, Mumbai | Zandu, Mumbai;DRF, Ghaz.; AVS, Kottakal; NRPL, Bangalore; LUPIN, Mumbai | 423.645 | |
| 13. Biotechnology for replacing chemical process in leather sector- Phase I & Phase II | CCMB; CLRI; NCL; IMT; IISc., Bang.; ARI, Pune; AU, Karaikudi; MKU, Madurai; UOP, Pune; DUSC, New Delhi; UOH, Hyd.; CDFD, Hyderabad.; SSF | - | 1689.850 | |
| 14. Improved granular processing: towards energy efficiency and resource conservation in cement manufacture | NCL; NML; IIT-B; NBCCM | ACC, Mumbai; L&T, Mumbai; GIL, Nagada; ICL, Chennai | 344.300 | |
| 15. Environmentally secure rare earth based colorants for surface coatings & Phase II | CLRI; RRL, Bhu; RRL, Trivd.; IISc.; IITM; ARI, Pune; Indian Rare Earths Ltd., Mumbai | Anabond Ltd., Chennai; Asian Paints Ltd., Mumbai; Gharda Chem. Ltd., Mumbai; Tata Refractories Ltd. | 276.285 | |
| 16. Functionalization of alkanes | NCL; IICT | IOCL; IPCL; RIL | 603.345 | |
| 17. Novel Molecular Diagnostics For Eye Diseases And Low Vision Enhancement Devices & Phase II | CCMB; IICB; IISc.; VRF, Chennai; LV Prasad Eye; AIIMS; CHG, Bangalore; GND Univ., Amritsar; Iladevi, Ahmedabad; Aravind Medical, Madurai; IITB; UOP, Pune | Xcyton, Bang.; LOPL; IOC, Baroda | 515.020 | |
| 18. Nano-Material Coatings And Advanced Composites For Tribological Applications In Automotive Industry | RRL, Triv; IISc.; ARCI, Hyderabad | TVS Motor, Chennai | 491.472 | |
| 19. Value added polymeric materials from renewable resources: Lactic acid and lactic acid based polymer | NCL; CFTRI; IICT; IMT; IIT-B | Godavari Sugar Mills Ltd., Mumbai; RGI, Mumbai; Prathista Biotech Ltd., Secunderabad | 1137.156 | |
| 2004-2006 | 20. Improved Genome Annotation Through A Combination Of Machine Learning And Experimental Methods: Plasmodium Falciparum As A Case Study | IGIB; CDRI; CDFD; IISc.; ISI, Kolkata; JNCASR, Bengaluru | TCS, Hyderabad | 596.467 |
| 21. Oral Delivery of Insulin | NCL; SCTIMST, Thiru.; NIPER | USV, Mumbai | 239.600 | |
| 22. Pharmacological and Genomic Investigations on Withania somnifera - An Indian Medicinal Plant (including bridging period of 2 years) & Leads based drug development and genetic improvement of Ashwagandha | CDRI; CIMAP; IICB; IICT; NBRI; RRL, Jammu | - | 1022.317 | |
| 23. Development Of Novel Fungicides | IMT; NCL; IICT; MS Univ., Baroda, NBRI | Rallis Res Centre, Bangalore | 485.122 | |
| 24. Biotechnological Approaches for Improvement of Plant Species with Special Reference to Pulp and Paper | NBRI; CIMAP; IHBT; NCL | Osmania Univ.; Lucknow Univ.; KFRI, Peechi; FRI; JK Paper Ltd; Ballarpur Ind. Ltd.; ITC Ltd. | 760.6868 | |
| 25. Development of fuel cells based on Hydrogen | NCL; CECRI; CGCRI; NPL; IICT; IIP; CMERI; NAL; RRL, Bho.; NML | - | 2050.338 | |
| Development of fuel cells based on Hydrogen- Phase II | NCL; CECRI; NPL | RIL, Mumbai | 1265.139 | |
| Demonstration and Validation of 3kWe PEMFC System | NCL; CECRI; NPL | RIL, Mumbai | 493.869 | |
| 26. Development of a 500 kW low cost horizontal-axis Wind Turbine | NAL; SERC | SGC, Coimbatore | 903.300 | |
| 27. Novel expression system | CCMB; RRL-Jam.; MKU; IIT-B | Biocon, Bang.; Shantha Biotech, Hyderabad | 259.6975 | |
| 28. Development of selected Medical Implants & Development of Dental Implants-Phase II study | CGCRI; CMERI; SCTIMST, Triv.; AIIMS; SSSIHMS, Bang.; MDDH&VI, ND; | TTK Healthcare; Universal Orthosystems, Vadodara; Genova Biotech, Hyd.; BBIL, Hyderabad | 1368.427 | |
| 29. Genetic Improvement of Jatropha curcas for Adaptability and Oil Yield | CSMCRI; NBRI; RRL, Jt.; NBPGR, N.D.; ICGEB, N.D.; Anand Agri.; CRIDA, Hyd.; FRI, Dehradun; AFRI, Jodhpur | Phytotron, Ban.; Excel Ind., Mumbai; JK Agri, Kolkata | 647.069 | |
| 30. Novel formulation for treatment of pulmonary tuberculosis – clinical studies | Lupin, Pune | 432.45 | ||
| 31. Process for Tamiflu – a Blockbuster drug to combat the menace of avian flu | NCL; IICT | - | 37.500 | |
| 32. Development of production system for Tea Polyphenols and their condensed products | IHBT; TRA, Jorhat; UPASI, Valparai; | Panacea Biotech Ltd | 253.798 | |
| 33. Development of an integrated micro PCR system with In-situ Identification - Phase II | RRL, Jammu; IISc, Bengaluru | Bigtec Ltd, Bangalore | 1035.510 | |
| 34. A prospective study to correlate gene signatures with clinical outcome of astrocytomas and identification of potential therapeutic target(s) | JNCASR, Bangalore; SSSIHMS, Bangalore; IISc., Bangalore; NIMHANS, Bangalore | Dabur India, Ghaziabad | 557.055 | |
| 2007-2009 | 35. Conversion of cellulose and hemi-cellulose into sugars and ethanol | IICB, Kolkata; IIT, Mumbai; IMT, Chandigarh; MKU, Madurai; NCL, Pune; RRL, Trivandrum; UDSC, New Delhi | - | 663.370 |
| 36. Conversion of Bioglycerol into value added chemicals | NCL, Pune; UICT, Mumbai; IICT, Hyderabad; IIT, Mumbai | Tata Chemicals; RIL, Mumbai | 430.270 | |
| Scale up of CSIR-NCL process for selective hydrogenolysis of glycerol to 1, 2- Propanediol- Phase 2 | NCL | Praj Industries Limited, Pune | 615.500 | |
| 37. Novel approaches for production of hybrid seeds with characteristics of improved insect resistance and higher yield | UDSC, New Delhi; IARI, New Delhi; NBRI, Lukcnow; TNAU, Coimbatore; BI, Kolkata; CICR, Nagpur; NII, New Delhi; UDSC, New Delhi; UAS, Dharwad | JK Agri-Genetics, Hyd.; Swagath Biotechnologies Pvt. Ltd., Hyderabad; Krishidhan Seeds Ltd., | 1458.477 | |
| 38. Meso-scale modeling for Monsoon predictions - Phase II | NAL; JNLCASR; IIT-B, Mumbai; TIFR Centre, Bangalore | ES, Bangalore | 2565.145 | |
| 2010-2013 | 39. Novel DPP IV inhibitors for the treatment of Diabetes | NEIST, Jorhat; CDRI, Lucknow | Cadila Pharma Ltd., Ahmedabad | 1481.46 |
| Phase II Project on DPP IV inhibitors | -- | Cadila Pharma Ltd., Ahmedabad | 699.67 | |
| 40. Biofuel from marine microalgae | Andhra Univ., Vis'tnam; Calcutta Univ., Kolkata; CSMCRI; IICT; IIT-Kharagpur; NCL; NIO; NIOT, Chennai; NIIST, Thiruvanathpuram | - | 1289.38 | |
| 41. Discovery and Validation of New Biomarkers for Head & Neck Cancer in India | IICB, Kolkata; PAKH, Mumbai | TCG Lifesciences Limited, Kolkata | 1111.32 | |
| 42. Design, Dev. and demonstration of high performance Parabolic Trough based 300 kW Solar Thermal Power Plant | IIT-M, Chennai; NCL, Pune; RRCAT, Indore | Millman Thin Film Systems Pvt. Ltd., Pune | 2436.87 | |
| 43. Dev. and demonstration of 500W SOFC Stack with hydrogen as fuel and testing of short stack with synthetic gas | CGCRI; NCL; IIT, Kharagpur | GAIL, R&D Centre, Noida | 550.16 | |
| 44. The next generation wind power technology: contra-Rotor Wind Turbine (CRWT) System | SERC; C-WET, Chennai | Wind Turbine System, Bang. | 1.056 | |
| 45. System based computational model of skin (SCoMOS) | NCL, JNU, NII, IGIB | Persistent Sys. Ltd., Pune | 687.6814 | |
| 2014-2019 | 46. Demonstration and validation of a 5KW HT-PEMFC based combined cooling and power system | NCL; CECRI, Chennai Centre; NPL | Thermax Ltd., Pune | 1526.82 |
| 47. Demonstration and validation of a LT-PEMFC system for Automotive application | NCL; CECRI, Chennai Centre | KPIT, Pune | 746.74 | |
| 48. Development of an antidiabetic agent based on the Phytopharmaceutical drug guidelines from Enicostemma littorale blume | MRC-KHS, Mumbai | Viridis Biopharma, Mumbai | 228.589 | |
| 49. Kappaphycus alvarezii and Red seaweed based formulations for improving productivity and health of dairy and poultry animals | CSMCRI, IITR; CARI, Izatnagar; NDRI, Karnal; IVRI, Izatnagar | Aqua agri Processing , ND | 372.8204 |
Annexure-5
NMITLI projects (Industry Originated Projects)
| Year | Project Title | Institutional Partners | Industry Partners | Budget (Rs in lakh) |
|---|---|---|---|---|
| 2000-2003 | 1. Development of an Oral herbal formulation for treatment of Psoriasis a clinical and scientific challenge - Phase I & Phase III | CDRI; NIPER | Lupin, Mumbai | 1671.166 |
| 2. Development of novel biotech therapeutic molecule –Lysostaphin & Development of novel biotech therapeutic molecule –Lysostaphin Phase II/III Clinical Studies | CDRI | BBIL, Hyderabad | 911.330 167.230 | |
| 3. Microbiological conversion of Erythromycin to Clarithromycin and other novel biologically active molecules | NCL; IMT; RRL, Jammu | Alembic, Vadodara | 107.660 | |
| 4. Recombinant approach to produce a-linolenic acid and docosahexanoic acid (DHA) in sunflower and yeast | NIO; IIIM, Jammu; IICT | Avestha Gen, Bangalore | 379.400 | |
| 5. A cost effective Simple Office Computing (Sofcomp) platform to replace PC | --- | Encore, Bangalore | 320.000 | |
| 2004-2006 | 6. A PC based high-end 3D visualization platform for computational biology – ‘Darshee’ | --- | Strand Genomics, Bangalore | 186.400 |
| 7. Development of Globally competitive ‘Triple-Play’ Broadband Technology | C-DAC, Pune | DiviNet, Pune | 1179.210 | |
| 8. Market seeding of SofComp and Mobilis to develop wide-ranging applications as well as increase awareness | --- | Encore, Bangalore | 537.00 | |
| 2007-2009 | 9. Development of high throughput marker assisted selection systems for improvement of drought tolerance and fibre quality related traits in cotton | NBRI; TNAU, Coimbatore | JK Agri-Genetics Ltd., Hyderabad | 1347.811 |
| 10. Novel method for development of B-type Natriuretic peptide (BNP) for diagnosis and treatment of congestive heart failure | IICT, Hyderabad; CCMB, Hyderabad | Virchow Biotech, Hyderabad | 465.270 | |
| 11. Development of Next Generation Plasma Display Technology and a 50 inch High Definition (HD) TV prototype | IIT, Kanpur; UOA, Allahabad; CGCRI, Kolkata; NPL, New Delhi | Samtel, Ghaziabad | 2478.17 | |
| 12. Wireless Sensor Network Chipset based on Ultra –Wideband Technology | _ | Virtual Wire Technologies | 422.00 | |
| 13. Design and development of cushion bonded organic ceramic clutch discs | IIT, Delhi; ARCI, Hyd.; NCL, Pune; CGCRI | Clutch Auto Ltd., Faridabad | 2164.310 | |
| 14. Development of Intelligent video surveillance server system | --- | Mindtree Ltd., Bangalore | 484.010 | |
| 15. Novel therapy for management of sepsis - Phase I & Phase II | NII, New Delhi; IISc., Bangalore; PGIMER, Chandigarh | Cadila Pharmaceutical, Ahmedabad | 2784.965 | |
| 16. A syndromic approach to diagnosis of infections: development of DNA macro-chips for simultaneous detection of pathogens causing AES (Acute Encephalitic Syndrome) and septicemia | CCMB; NIMHANS, Bangalore; VRF, Chennai | St. John's MC&H, Bangalore; Xcyton, Bangalore | 1136.470 | |
| 17. Development of rice resistant to the fungal blast disease | - | Metahelix Life Sc. Ltd, Bengaluru | 83.270 | |
| 18. Development and characterization of an indigenous vaccine for Johne’s disease | CIRG,Mukhodoom; BBF, Hyderabad | Biovet Pvt. Ltd., Bengaluru | 574.900 | |
| 2010-2012 | 19. Development of caerulomycins as novel immunosuppressive agents to prevent organ rejection after transplantation and to address various autoimmune and allergic disorders | IMT, Chandigarh | EnEm Nostrum Remedies Pvt. Ltd., Mumbai | 1210.00 |
| 20. Development and production of a therapeutic monoclonal antibody against eNAMPT, a novel inflammatory target | USDC | Gennova Biopharmaceuticals Ltd. Pune | 2299.13 | |
| 21. Development and commercialization of “Vennfer” – unique H.264 high definition software based multiparty, multipoint video conferencing solution on multipoint network transmission protocol | --- | Intellisys Technologies & Research Ltd, Kolkata | 296.000 | |
| 22. Customized adaptation of nonClonableID technology to establish authenticity of medical products | --- | Bilcare Ltd., Pune | 893.46 | |
| 23. Development of a diagnostic system for affordable, point of need testing to manage HIV and TB | --- | Reametrix India Pvt. Ltd., Bengaluru | 816.08 | |
| 24. Development and commercialization of NXR-4D: A lithium-Ion battery powered 4-door next generation electric Car (4-Seater Capacity) | CECRI, CMERI | Mahindra Reva Electric Vehicles Pvt. Ltd., Bangalore | 1919.156 | |
| 25. Design and fabrication of All-Fiber Supercontinuum Light Source with application demonstration on spectroscopic signature detection | CGCRI | Vinvish Technologies Pvt. Ltd., Thiruvanathpuram | 282.75 | |
| 26. Development and commercialization of Soleckshaw Lite – An Innovative Electrical Green Transport Platform | CECRI, CMERI | Kinetic Engineering Ltd., Pune | 1410.978 | |
| 27. Development of non-hazardous process for the synthesis of Di Methyl Carbonate (DMC) from methanol and urea | NCL, IIT-B | Deepak Fertilisers and Petrochemicals Corp. Ltd., Pune | 409.489 | |
| 2013-2015 | 28. Development of integrated technological solutions for security and operations based on UV sensor technologies | CEERI Chennai Centre, CSIO | Aron Universal Ltd., Bengaluru | 259.216 |
| 29. Design and development of photonic crystal cladded and double called Er and Er/Yb fibers, and application demonstration of high-power optical amplifier | CGCRI | Vinvish Technologies Ltd., Thiruvananthapuram | 250.00 | |
| 2016-2019 | 30. Automation of Ghani operation through vacuum conveying system | -- | Fare Labs Pvt. Ltd., Gurgaon | 481.67 |
Annexure-6
Output under NMITLI projects
| S.No. | Project Title | Technology Developed | Knowledge base | No. of Patent and /or Publications |
|---|---|---|---|---|
| 1. | Two orders of magnitude improved liquid crystals for flat panel display devices | • The knowledge gained couldnot be translated for industrial purpose as the expertise for developing graphic display system was unavailable at that time. | • Knowledge on development of display system with improved characteristics; • New LCD materials were synthesized, characterized and developed; and • A prototype of 16 characters, single-line alphanumeric device developed. | Patents: 6, Publications: 2. |
| 2 | Defunctionalization of carbohydrates as a feed stock to manufacture well identified industrial chemicals | · The process could not be commercialized as it was beyond the scope of NMITLI and for other process the change in market dynamics made the process not viable. | • Developed a process for 5-hydroxy methyl furfural. But - requires huge effort to commercialize -beyond scope of NMITLI; • Developed a novel process for (S)3-hydroxy-g- butyrolactone. | One international and one Indian patent obtained. |
| 3 | New targets and markers for cancer using genomics and proteomics | --- | · Several differentially expressed genes and protein biomarkers identified from oral cancer tissues that can be exploited for diagnostic purposes. | • Patent filed on these markers. |
| 4 | Stimuli sensitive polymeric nano-particle based advanced drug delivery systems for cancer, diabetes and anti-bacterials | Technical hurdles faced while developing virosome and nanoparticle based drug delivery systems for anti-cancer and anti-bacterial drugs—Project component closed | · POC established for inhalation delivery of anti TB drugs | Three patent filed. |
| 5 | 5 & 25 KW decentralized power packs | The technology however could not become commercially viable. | · A prototype of integrated 5 kW Fuel Cell was developed, installed and demonstrated. | Patent filed: 4 Publications: 4 |
| 6 | Latent M. Tuberculosis: New Targets, Drug Delivery system, bio-enhancers and therapeutics | --- | · A new bioenhancer as an adjunct to chemotherapy established for latent tuberculosis infection; · 4 promising leads identified as candidates for IND | -- |
| 7 | Using Functional genomics in tea, mentha, ashwagandha, plants for gene expression modulation | · Agrotechnologies for New plant varieties of Mentha Pipereta ‘CIM-Indus’ & ‘CIM-Madhuras’ released to farmers; · A kit for catechin estmation | · Bidirectional and two element dependent novel gene expression vectors; · Tea database profling for catechins; · Agrobacterium mediated genetic transformation system. | Publications:42 Patents:Filed – 3, Granted – 6 |
| 8 | Meso-scale modeling for monsoon predictions II | · Developed and demonstrated integrated weather prediction platform consisting of 128 nodes parallel processor supercomputer with Floswitch and Flo-opti link for inter-processor communication; · Integrated Platform for monsoon prediction developed under CSIR-NMITLI & MoES | · A new weather prediction code Varsha 1.0 with physics relevant to the tropics ported on computer; · The platform was further upgraded by developing and demonstrating a 10 Teraflop Supercomputing Hardware Machine (Mk8) and improved software code (Varsha 1.1) with a higher resolution of 40 / 20 Km. The hardware machine consisted of many indigenously developed components, like new FPGA based FloSwitch etc. | --- |
| 9 | Nano-material catalysts and associated process technology for alkylation/ acylation/nitration of well identified industrial chemicals, pre-reforming of hydro-carbons and sulphur removal (<50 ppm) from petroleum fuels | · Technology could not be developed for commercial scale | · Process for removal of sulphur from petroleum fuels demonstrated; · Catalysts developed for pre-reforming of hydrocarbons | Patents: 3 |
| 10 | Versatile, portable PC based software for bioinformatics & Development of Linux cluster version of Bio-suite | Two bioinformatics softwares developed and marketed (i) BioSuite, both cluster version and stand alone; and (ii) Genocluster | -- | • Patents: Filed -2 • Publications: 10 |
| 11 | Biodegradable plastics from agricultural wastes: Cellulose esters based on bagasse & Steam explosion-based fractionation process of sugarcane bagasse to cellulose, hemicellulose and lignin-Validation at semi-commercial level | A world class technology for fractionation of sugarcane bagasse into cellulose, hemi-cellulose and lignin developed and licensed to M/s Godavari Biorefineries Ltd. | -- | US Patent granted |
| 12 | Herbal based preparations for degenerative disorders: Diabetes mellitus type II, Osteoarthritis and Rheumatoid arthritis and Common hepatic disorders | -- | Diabetes · Single herbal formulation developed and validated through limited multi-centric clinical studies in patients uncontrolled on existing drugs: sulphonylurea and metformin. Arthritis · Polyherbal formulation comprising 3 herbs developed and validated through large-scale multi-centric clinical studies. Hepatocellular Protection · Polyherbal formulation comprising 12 herbs developed and validated through limited multi-centric clinical studies. | · Publications: 25 on Arthritis component · Arthritis Patent granted in Europe--Great Britain and Denmark |
| 13 | Biotechnology for replacing chemical process in leather Sector-Phase-I & II | · Technology for Bio-processing of Leather (beam house operations). · A total of 5 proteases and 2 lipases have been developed and up-scaled for dehairing, degreasing and fibre opening along with alpha-amylase (other source). · Technology demonstrated by CLRI in tanneries. | -- | 1 Patent granted. |
| 14 | Enhanced productivity in cement manufacture through improved granular processing and resource conservation | -- | · Developed computational fluid dynamics based model of rotary cement kiln; · Demonstrated utilization of slag and flyash in preparation of Portland Slag Cement | 18 Publications; 4 Indian patents; 1 US patent; 1 Canada patent |
| 15 | Development of an Oral herbal formulation for treatment of Psoriasis a clinical and scientific challenge & Clinical studies of an oral herbal formulation for treatment of psoriasis & Phase-IIb & III Clinical Trials | -- | · Formulation developed for amelioration of Psoriasis; · However it failed to show efficacy in Phase III clinical trial in comparison to standard drug, methotrexate. | -- |
| 16 | Development of novel biotech therapeutic molecule –Lysostaphin & Phase-II Clinical studies: Novel bio-therapeutic molecule-Lysostaphin & Novel Biotherapeutic Molecule: Lysostaphin – Phase IIb/III Clinical studies | -- | · Developed lysostaphin gel formulation for Staphylococcus aureus infection; · Developed novel downstream process for large scale production of >99% pure protein; · However Phase III clinical trial couldnot show improved efficacy as compared to standard treatment available. | The IP on the molecule is protected through PCT with product patent in 85 countries, including USA. |
| 17 | Microbiological conversion of Erythromycin to Clarithromycin and other novel biologically active molecules | --- | · Project attempted to isolate microorganism that can convert biological active molecules to antimicrobials but failed in its attempt. | --- |
| 18 | Environmentally secure rare earth based colorants for surface coatings & Phase II | --- | · Forty-two color shades based on rare earth compounds and selected dopants were developed for use in pigment applications for surface coatings; · Innovative combustion synthesis based route for cerium sulphides and oxysulfides and lanthanum sulphide to develop new range of colors; · Sulfate reducing bacteria based method developed that showed promise in securing environment. | 1 US patent granted |
| 19 | Functionalization of alkanes | --- | · A modified catalytic process for economical production of acetic acid and ethylene from ethane was developed; · A modified catalytic process for production of vinyl chloride monomer from ethane was developed. | 2 patents granted |
| 20 | Novel molecular diagnostics for eye diseases and low vision enhancement devices & Development of DNA Macroarray for the detection of eye infections and the genetic predisposition to glaucoma | · Three low vision enhancement devices developed viz. a) The Illumination Table with Variable Spectral Distribution and Intensity b) Night Vision Pocketscope and c) Spectacle mounted prismatic stereovision lenses of plastic with anti-scratch coating. · Diagnostic system for detection of eye infection commercialized. | · A macrochip containing 4 candidate gene to facilitate easy and rapid diagnosis and clinical characterization of individual eye has been developed. | --- |
| 21 | Nano-material coatings and advanced composites for tribological applications in automotive industry | --- | · ROTOPLASMA XPT 512 coated cast iron sleeve cylinder blocks for reduced oil consumption, reduced wear rate and retention of surface finish compared to regular cast iron cylinder liners were developed; · Aluminium alloy matrix hybrid composite containing silicon and graphite particles were developed, synthesized and characterized; · A process for making functionally graded aluminium matrix composite cylinder liners was developed. | 12 Publications 1 Indian patent granted. |
| 22 | Value added polymeric materials from renewable resources: Lactic acid and lactic acid based polymers & Lactic acid and lactic acid based polymers-establishment of 300 TPA pilot plant for lactic acid production | · Process for production of lactic acid (3000 litre capacity) from fermented sugarcane juice by lactobacillus and its separation as calcium lactide was demonstrated at industry site, M/s Godavari sugar mills Ltd; · Technology however could not be made commercially viable due to market conditions. | · A mutant strain of lactobacillus delbrueckii MAC 168701 isolated that can produce L-lactic acid; · A lab scale (100 l) fermentation process demonstrated for production of highly pure lactic acid from sugarcane juice; | ---- |
| 23 | Recombinant approach to produce a-linolenic acid and docosahexanoic acid (DHA) in sunflower and yeast | § A technology for the production of DHA from fermentation of thraustochytrids species SC-1 has been developed and transferred to industry partner for commercial exploitation. | · Recombinant approach for production of DHA was attempted but was not successful. | ---- |
| 24 | A cost effective Simple Office Computing (Sofcomp) platform to replace PC | · Three variants of SofComp devices developed. | -- | Copyrights: 3 |
| 25 | A PC based high-end 3D visualization platform for computational biology – ‘Darshee’ | · A low cost visualization platform for bioinformatics with features of portability, scalability and upgradability developed. · Product marketed as part of Industry’s Avadis platform; · The technology was further integrated into Array Assist of Stratagene, USA. | --- | --- |
| 26 | Improved Genome Annotation Through a Combination of Machine Learning and Experimental Methods: Plasmodium falciparum As a Case Study | ---- | · Algorithms and softwares developed for predicting, identification and annotation of genes in prokaryotes using P. falciparum as case study; · The genes predicted using the softwares developed have been experimentally verified using gene expression studies | --- |
| 27 | Oral Delivery of Insulin | --- | · Development of oral insulin formulation was attempted but was not successful. | 1 patent has been granted. |
| 28 | Pharmacological and Genomic Investigations on Withania somnifera - An Indian Medicinal Plant & Leads based drug development and genetic improvement of Ashwagandha (Withania somnifera) | · Five chemotypes namely, NMITLI 101, NMITLI 108, NMITLI 118, NMITLI 128 and NMITLI 135 and a phytochemical hybrid (NMITLI 101XNMITLI 135) were developed; · NMITLI 118 variety released to farmers. · Agrotechnologies for NMITLI Ashwagandha varieties developed; · NMITLI 101 variety released to farmers. | · Indian chemotype varieties developed and fingerprinted; · Pharmacological activities of different varieties deduced; · Marker compounds from Ashwagandha root extracts developed as phytochemical standards; · Ashwagandha monograph publishe · Antistroke activity proved using NMITLI 118 extract in animal models; · NMITLI 101 extract proved to be best immunomodulator clearing more than 90% Leishmania and Candida infections in animal models. | 1 US patent granted. More than 20 publications 3 patents filed; 43 publications |
| 29 | Development of Novel Fungicides | --- | · Novel fungicides synthesized which have strobilurin and 1,2,4 triazoles unit in them showed activity against pathogenic fungi at 25 ppm.; · Suitable formulations developed and they were subjected to field trial by industry; · Targets for few compounds were identified for their mechanism of action; · Antifungal compounds were isolated from microbes (Psuedomonas sp) growing in extremophilic and mesophilic environment; · Industry partner deployed these fungicides in their field as a part of trial. | 1 Indian patent granted and a PCT application published in few countries |
| 30 | Biotechnological Approaches for Improvement of Plant Species with Special Reference to Pulp and Paper | --- | · Project efforts led to rich collection of Ochlandra germplasm being maintained at KFRI, Peechi which has low lignin content suitable for paper industry; · Eight elites of Leucaena lecocephala (subabul) havng high fibre length and low lignin content are being maintained at NBRI; · Micropropagation techniques and regeneration system for mass multiplication developed for these varieties which are useful for paper industries. | ---- |
| 31 | Development of fuel cells based on Hydrogen & Development and demonstration of polymer electrolyte fuel cell stacks for stationary applications & Demonstration and Validation of 3kWe PEMFC System | A prototype of 1.00 kW based on the Polymer Electrolyte Membrane Fuel Cell (PEFC) technology has been designed, developed and demonstrated. | · Knowhow for modified membrane for the operation of PEMFC at low relative humidity and high temperature;· A modular 3kWe PEFMC test bed designed, built and commissioned at RIL’s Patalganga site where adequate amount of hydrogen is available. | --- |
| 32 | Development of a 500 kW low cost horizontal-axis Wind Turbine | 500 kW horizontal-axis wind turbine has been designed, fabricated and successfully commissioned at the test site, Kethanur, Tamil Nadu | ---- | --- |
| 33 | Novel expression system | ---- | · Five novel promoters were developed that drive the production of some industrially important enzymes; · Five novel yeast species were identified from varied sources; · Alcohol oxidase promoter were developed from novel alcohol oxidase genes cloned from a yeast strain. | One Indian patent |
| 34 | Development of selected Medical Implants And Development of Dental Implants-Phase II study | Dental implants of sizes based on anthropometric measurement for Indian patients and complete surgical accessories developed; Technology transferred to M/s IHPL; Dental implants commercialized in the nameof ifix by KMPL, Faridabad. | · Prototype for titanium nitride coated drug eluting cardiovascular stents were designed and prototypes fabricated; · Ceramic based femoral head, acetabular cup and ceramic hip prosthesis developed and trial was done in pvt clinical centers; · Hydoxyapatite and bioglass coating of orthopaedic implants developed. | One US patent and One Indian patent on dental implants obtained. |
| 35 | Genetic Improvement of Jatropha curcas for Adaptability and Oil Yield | · 185 accessions of Jatropha collected from wild were systematically characterized and genotypically mapped; · Germplasm bank established at NBRI and NBPGR Ranchi; · Agrotechnology for plant establishment in place through multilocational trial; · Genetic transformation methods developed using stress related gene from plant. | · Patents filed: 2 · Publications: 36 | |
| 36 | Development of Globally competitive ‘Triple-Play’ Broadband Technology | The triple play technology has been successfully developed and tested using the BSNL broadband infrastructure to provide end-to-end solution for triple play services. BSNL was fully satisfied with the technology and adopted it for commercialization. | --- | An Indian patent (No. 201179) granted. |
| 37 | Novel formulation for treatment of pulmonary tuberculosis – clinical studies | --- | The formulation from the molecule did not showed desired protection in TB patients as compared to protection seen in animal model. | Patents: 3 |
| 38 | Market seeding of SofComp and Mobilis to develop wide-ranging applications as well as increase awareness | · 2000 units were produced for deployment at customer end for evaluation · Exclusive license for manufacturing and marketing given to an industry, DSK-Worldman Computers Pvt Ltd, Pune. | --- | ---- |
| 39 | Process for Tamiflu – a Blockbuster drug to combat the menace of avian flu | --- | A 12 step process for production of tamiflu starting from (-) shikimic acid developed. | ---- |
| 40 | Development of production system for Tea Polyphenols and their condensed products | · Green process developed for extraction and purification of catechins—amino acids as by-product; · Process upscaled to 40kg leaves/batch · Technology transferred to Baijnath Pharmaceuticals Pvt Lt, Paprola, HP | · New genes for catechin biosynthetic pathway from tea plant found that were registered with NCBI; · Polyphenol oxidase (PPO) gene in catechin biosynthetic pathway cloned and expressed; · Qualitative screening of tea for clonal variation in PPO activity undertaken in 700 tea accessions. | · Publications: 9 · Patent Granted in USA & China |
| 41 | Development of an integrated micro PCR system with In-situ Identification & Validation of handheld diagnostic platform for HBV detection | Technology developed for · Micro PCR Device · Sample Preparation Device · Microchips for detection of Tuberculosis, Malaria, Dengue, Chikungunya, Hepatitis B and H1N1 Commercialized by M/s Bigtech Pvt ltd | ---- | 70 patents granted. |
| 42 | A prospective study to correlate gene signatures with clinical outcome of astrocytomas and identification of potential therapeutic target(s) | --- | · Identified glioblastoma biomarker genes; · Shortlisted genes from microarrays as potential diagnostic marker genes | --- |
| 43 | Development of high throughput marker assisted selection systems for improvement of drought tolerance and fibre quality related traits in cotton | --- | · More than 2000 SSR markers were identified in cotton which were developed in Gossypium hirsutum genotype for future R&D in improving cotton varieties; submitted in cotton marker database; · Recombinant inbred lines of one variety developed that showed best performance for yield and quality parameters. The line was included in multilocational trial program of TNAU, Coimbatore. | 3 Publications |
| 44 | Novel method for development of B-type Natriuretic peptide (BNP) for diagnosis and treatment of congestive heart failure | A prototype diagnostic device for detection of Congestive Heart Failure was successfully developed | · A novel expression system that can produce large quantities (10mg/L) of highly pure rhBNP for therapeutic or for diagnostic usage was developed; · The purified rhBNP was characterized for its purity and activity and safety in animal studies; · The knowhow for liposomal encapsulated formulations generated. | 2 Publications |
| 45 | Development of Next Generation Plasma Display Technology and a 50 inch High Definition (HD) TV prototype | A prototype of 50’’ HD PDP with existing materials / processes including its electronics was designed, developed and demonstrated. | ---- | Patents filed: 19; Publications: 18 |
| 46 | Wireless Sensor Network Chipset based on Ultra –Wideband Technology | · Prototype (4x4 MIMO UWB) developed · Working prototype for transferring data at the rate of 1 Gbps wireless link using a non-standard protocol (3 GHz band) demonstrated | --- | 3 patents filed |
| 47 | Conversion of cellulose and hemi-cellulose into sugars and ethanol | --- | · Microbial consortia for production of various enzymes; · Scale up process for enzyme production | ---- |
| 48 | Conversion of Bioglycerol into value added chemicals & Scale up of CSIR-NCL Process for selected hydrogenolysis of glycerol to 1, 2-Propan-di-ol | Technology for conversion of glycerol into 1, 2-PDO developed. | --- | A US patent has been granted. |
| 49 | Novel approaches for production of hybrid seeds with characteristics of improved insect resistance and higher yield | ----- | Rice component · Seeds & data (morphological & molecular) on 566 rice genotypes · Diverse parental lines for inter-varietal crosses – 200 identified as promising · 168 diverse restorer lines · 200 hybrids out of which 21 found promising – 5 as most promising · 380 promising iso-cytoplasmic F7 restorer lines from 25 most promising commercially cultivated hybrids Cotton component • Barnase male sterile lines • Barstar male sterile lines • cry1Ec construct with novel promoter & transgenic lines • cry2Ax1 construct & transgenic lines • AMTL lectin construct • CEA lectin construct • 1 Barbadense pure line • 4 hybrids • 4 combiners each from 4 heterotic groups | Patents: 3; Publications: 8 |
| 50 | Meso-scale modeling for Monsoon predictions - Phase II | Same as Sr. No. 8 | --- | --- |
| 51 | Design and development of cushion bonded organic ceramic clutch discs | Cushion Bonded/ Rigid Bonded Organic, Ceramatallic Cookie & Single/Dual Sintered Buttons (Copper / Iron Based), Ceramic Cookie & Annular Ring Clutch Discs and Matching Cover Assemblies’ have been developed | 5 Patent filed 14 Design Registered | |
| 52 | Intelligent video surveillance system | Intelligent video surveillance solutions comprising of flexible and reliable Digital Video Recorder (DVR), video decoder and the open network video interface forum (ONVIF) stack developed and commercialized. | --- | 4 Publications 3 US patents granted |
| 53 | Novel therapy for management of sepsis and Novel therapy for management of sepsis Phase II study | · The drug is already in market for other indication. · For management of sepsis—awaiting marketing approval for new indication for management of gram negative sepsis | · Phase IIa clinical trial for safety dose showed faster clinical, functional and microbiological recovery when combined with standard therapy; · Phase IIb/Phase III multicentre clinical trial completed and there was 10.89% absolute reduction and 55.56% relative reduction in mortality in Treatment Group compared to Control Group which is clinically relevant and statistically significant (p = 0.0229). · The survival data of died patient’s shows that there is significant difference between two arms for the days of death. Death was delayed in the treatment group compared to control group. [p-value: 0.0001, Hazard Ratio: 0.448 (95% CI: 0.311-0.646)]. · Product developed and named “sepsivac”and is commercialized by M/s Cadila Pharmaceuticals Ltd, Ahmedabad | One product patent |
| 54 | A syndromic approach to diagnosis of infections: development of DNA macro-chips for simultaneous detection of pathogens causing AES (Acute Encephalitic Syndrome) and septicaemia | The project was successful, culminating with the development of three diagnostic kits – two for simultaneous identification of all pathogens causing Acute Encephalitic Syndrome and septicaemia and the third one for identification of the antibiotic sensitivity of the bacteria causing septicaemia. | ---- | ---- |
| 55 | Evaluation of RNAi-based constructs for conferring resistance on transgenic rice against the blast fungus Magnaporthe grisea | --- | · Transgenic rice plants of T5 generation were developed that showed good level of resistance against blast fungus; · Technology platform for developing transgenic rice plants ready with gene overexpression and gene silencing targets for clast fungus in place. | ---- |
| 56 | Development and characterization of an indigenous vaccine for Johne’s disease | Two formulations of vaccine i.e. JD Oil and JD Gel and companion diagnostic for JD developed. | --- | Patent filed: 2 |
| 57 | Development of Caerulomycins as novel immunosuppressive agents to prevent organ rejection after transplantation and to address various auto-immune and allergic disorders | Analogues of original molecule, Caerulomycin, an immunosuppressive agent for organ rejection, were developed and tried for activity and IND studies; However the selected analogues did not show the activity of immunosuppression and the project was closed. | 1 Patent filed; 5 publications | |
| 58 | Novel DPP IV inhibitors for the treatment of Diabetes & Novel DPP IV inhibitor – Phase I/II Study: A safety, pharmacokinetic and pharmacodynamics study of CPL-2009-0031 in Healthy Volunteers and patients with Type 2 Diabetes mellitus (T2DM) | · One novel DPP IV inhibitor identified from among synthesized molecules; · Novel process developed for synthesis of the molecule; and · Drug development related pre-clinical studies on the selected molecule for IND completed. · In Phase-I study, the drug was found to be safe and well tolerated at all dose levels, without any major safety concerns in healthy volunteers. · Additionally, it demonstrated linear pharmacokinetic characteristics after oral administration. · Phase-II prospective, randomized, double blinded, parallel group, multicentric, comparative clinical study indicated efficacy of the compound. No deaths, other SAEs or other significant AEs were observed in any of the patients during the entire study · Based on the Phase-II data, the Monitoring Committee suggested comparative study of the drug with Sitagliptin in phase III clinical trial. The proposal has been approved by DCGI. | · Patent: 3 PCT (1 patent granted in 5 countries; 1 granted in 2 countries); 1 filed in India and Europe | |
| 59 | Biofuel from marine microalgae | · Novel process developed for harvesting of micro algal biomass. · Process developed for biodiesel production from a marine microalgal species at cost ~ Rs 125 per litre. Process patent granted. | · Publications: 64 · Patents: Filed-1; Granted-5 | |
| 60 | Development and production of a therapeutic monoclonal antibody against eNAMPT, a novel inflammatory target | · Ultra-large naïve human phage display libraries (2.79 x 1011 cfu total); compare with Xoma’s (2.5 x 1011 cfu total); · Fast ultra-large library making abilities – 30-45 days from start to sequence characterization; · Proof-of-concept of practical applicability of antigen-antibody equilibrium and kinetic principles for phage panning and isolating high affinity phage pools; · Fast panning – 21-30 days for 4 rounds of solution panning to monoclonal isolation; · Emphasis on studying monoclonals as Fab proteins in solution (not fusion with phages) and novel solutions for phenotyping to isolate “genotype true” hits; and · Establishment of novel solutions for kinetic ranking of Fab hits as proteins. | Antibody Phage Display Library’ filed on 23rd June, 2017 with the ID PCT/IN2017/050257 | |
| 61 | Biomarker Neck and Head Cancer | --- | · Robust data generated which include complete clinical analysis combined with transcriptome profiling of 350 head and neck cancer (HNCC) patients’ samples The probable markers can now be visualized that can be considered for making chip based diagnostic prototype; · A prediction algorithm has been developed which gives prediction of more than 95% accuracy. The 83 gene set showing strong prediction power of HNCC occurrence can now be superimposed for multivariate clinical analysis. Thus a proof of concept with patients’ samples has been established; · A signature of 34 transcripts correctly predicted 41 of 50 samples (82% Prediction accuracy) in the validation phase. Prediction analysis based on PNI status and tumor size showed prediction similar to the whole sample. The 34 transcript signatures are novel, specific for Indian population and do not overlap with 102 gene signature obtained in the Dutch study. | A PCT filed for the biomarkers. |
| 62 | Design, Development and demonstration of high performance parabolic trough based 300 kW Solar Thermal Power Plant | ---- | · A new Magnetron Sputtering Pilot Plant System which is capable of co-deposition and as well sequential deposition of six different types of coatings to address the need for various functionality of (a) IR reflection, (b) Diffusion barrier, (c) Multilayer cermets, (d) Multilayer antireflection coating etc.; · Tracking System with features: (i) Hydraulic System coupled with Advance Controller (ii) Inclinometer for absolute measurement of position (iii) Drives 6 modules of three Troughs on each side (iv) Accuracy 1.7 mrad (v) Validation at DLR, Germany is underway; · Photo-receiver Tube (Heat Collection Element) for 4 m size with measurement of: (i) Thermal loss (ii) Assess performance in outfield natural Solar Radiation (iii) Vacuum leak test (iv) Vacuum integrity with time and (v) Optical efficiency test on trough; validation to be done at DLR, Germany; · Highly engineered Concentrating Parabolic Trough Collector assembly with unique features was designed, developed and demonstrated; and · A high precision Glass to Metal Seal with High Temperature stability for Heat Collection Element developed and integrated into the HCE as per the satisfaction of industry. | ---- |
| 63 | Development and demonstration of 500 W SOFC Stack with hydrogen as fuel and testing of short stack with synthetic gas & Development and demonstration of 1 kWe SOFC Stack with hydrogen & Air and 500 We SOFC Stack with reformed natural Gas and Air | Not yet commercialized. | Developed and demonstrated 3 different prototypes of capacities 200W, 500W and 1.0 kW of Solid Oxide Fuel Cell Stack using hydrogen and Air+O2 with peak power density of 0.31 W/cm2 at 800oC. The other achievements have been the development of thermally cyclable new glass sealants for SOFC stack and theoretical modeling of the cell design. | Patents: 3 & Publications: 15 |
| 64 | The next generation wind power technology: contra-Rotor Wind Turbine (CRWT) System | Projects foreclosed | --- | --- |
| 65 | Development and commercialistion of “Vennfer” – unique H.264 high definition software based multiparty, multipoint video conferencing solution on multipoint network transmission protocol | High definition software based video conferencing solution – ‘Vennfer HD’ using H.264 video codec multiparty, multipoint video conferencing applications on multicast network transmission protocol has been developed. | --- | --- |
| 66 | Development and commercialization of NXR-4D: A lithium-Ion battery powered 4-door next generation electric Car (4-Seater Capacity) - Mahindera Reva Electric Vehicles Pvt. Ltd., Bangalure | First four-door electric vehicle to be entirely indigenously designed, developed and commercially produced in India and meeting global automotive quality standards | --- | --- |
| 67 | Design and fabrication of All-Fibre Supercontinuum Light Source with application demonstration to detect fake pills: Vinvish Technologies Pvt. Ltd., Thiruv’puram | Developed and Demonstrated two products viz.: (i) Supercontinuum Light Generation Source; and (ii) Broadband Confocal Microscope. The developed PCF technology had been transferred to the industrial partner by CSIR-CGCRI through a Technology Transfer Agreement. | --- | ---- |
| 68 | Development and commercialization of Soleckshaw Lite – An Innovative Electrical Green Transport Platform: Kinetic Engineering Ltd., Pune | · Indigenous Electrical three wheeler. · Central Motor Vehicle Rules (CMVR) certification for the same has been obtained | --- | --- |
| 69 | Customized adaptation of nonClonableID technology to establish authenticity of medical products: Bilcare Ltd., Pune | The nonClonableID technology was demonstrated for its utility in: (i) establishing the product accountability in the area of medical products; (ii) secured traceability from the point of origin to the point of dispensation, authenticity check and establishing e-pedigree; and (iii) improving medication compliance by patients | ---- | ----- |
| 70 | Development of a diagnostic system for affordable, point of need testing to manage HIV and TB: Reametrix India Pvt. Ltd., Bangaluru | · ReaSLR CSM Kit Ver 1.0 (ReaFilter based filtration and Concentration of cells by centrifugation) - is available for evaluation and sale; | · ReaSTAT CD4 assay on IMMEDIA - External validation of ReaSTAT CD 4 assay kit is ongoing; · ReaSLR CSM Kit ver 2.0 (magnetic beads and staining) - External validation is being carried out at three centers; and · ReaTBDx – Antibody licensed from Albert Einstein School of Medicine, New York; Optimization of is being carried out at SGPGIMS Lucknow; External validation of ReaTBDx will be carried out at external site. | --- |
| 71 | Development of non-hazardous process for the synthesis of Di Methyl Carbonate (DMC) from methanol and urea | Developed two-step process for the synthesis of dimethyl carbonate (DMC) from methanol and urea | · Developed Two Step Process for the synthesis of DMC from methanol and urea. · Novel reactor configuration proposed for both the steps. · Protocol standardized for regeneration of the used catalyst · Demonstrated Process at Bench Scale Set-up (45 gm/hr); · Demonstrated the process for synthesis of other higher dialkyl carbonates from corresponding alcohols · Filed six Patents covering Catalysts, Process and catalyst regeneration strategies | Publications: 1; Indian Patent granted : 1 |
| 72 | System based computational model of skin (SCoMOS): CSIR-IGIB, Delhi | Computational software named “eskin” encompassing varied features as mentioned in next column launched by industry for services. | · A unique repository of 2600+ skin specific genes and their associated skin pathways obtained by manual curation of biomedical literature (called Skin base). · Detailed molecular interaction maps of 35 skin physiology associated biological pathways (e.g. cornification, keratinocyte differentiation); · Offers skin centric analysis of gene expression data with overlay functionality for easy identification of pathways most relevant to a phenotype; · Offers Functionality for comparing unknown compound’s gene expression signature with those related to skin-specific phenotypic endpoints (e.g. hypopigmentation, desquamation)—Skin predict · Facility for extensive reporting module to generate generic as well as customized reports for the analyses performed; and · User-friendly interface and optimized architecture for computation-intensive jobs; · Gene Signatures expressed by human skin in response to ten compounds of five different groups i.e. hyperpigmenting, hypopigmenting; hydrating; photo-oxidative and desquamation that are commonly used by cosmetic and pharma industries in their products, were generated and incorporated in the software. | Copyright for software |
| 73 | Integrated technological solutions for security and operations based on UV sensor technologies | Few developed material products have been commercialized by M/s Security Printing and Minting Corporation of India Limited (Wholly owned by Govt. of India). | · Developed Invisible UV sensitive inks fluorescing in blue color as per the industry standards; and · Six different Pi-MAD (Programmable Invisible Marker Authentication device) prototypes with varying functionality have been developed leveraging open source Arduino platform which can clearly distinguish various fluorescent colors. | --- |
| 74 | Design and development of photonic crystal cladded and double called Er and Er/Yb fibers, and application demonstration of high-power optical amplifier | A prototype of high power amplifier (EDFA) was designed, developed and demonstrated. | --- | --- |
| 75 | Demonstration and validation of a 5 kW HT-PEMFC based combined cooling and power system | Not yet commercialized. | · 6.0 kW Fuel Cell Test Station from Lean Cat has been installed and commissioned; · 3.0 kW Fuel Cell Stack with 100 Cells has been built; · Considerable progress has been made on the aspects of Reformer, Power Conditioner, BoP components, System Integration and Packaging of the system; and · Design of Vapor Absorption cooling system initiated. | Patent Filed: 1 Publications: 10 |
| 76 | Demonstration and Validation of a LT-PEMFC system for automotive application | Demonstration of hydrogen fuel cell prototype in running the sedan car. | · A 30-Cells stack has been built using the MEA that were created by hot pressing the electrodes fabricated. | Publications: 1 |
| 77 | Development of an antidiabetic agent based on the phytopharmaceutical drug guidelines from Enicostemma littorale blume | --- | Four markers have been identified for development of phytopharmaceutical formulation | --- |
| 78 | Kappaphycus alvarezii and red seaweed based formulations for improving productivity and health of dairy and poultry animals | · Two formulations developed and validated ü One formulation for improving health including immunity enhancement and productivity of broiler chicken ü Another formulation for improving health and immunity of crossbred calves and dairy cattle as also having anti-methanogenic property. | --- | Publications : 2 |
| 79 | Automation of Ghani operation through vacuum conveying system | Fully automatic Ghani plant has been designed, fabricated, installed and commissioned at M/s Khandelia Oil Mills, Alwar. | Complete package for conversion/retrofitting of traditional ghani plant into fully automatic ghani plant. | Patent Filed : 1 |
Annexure-7
List of Major Technologies/Process Developed under CSIR-NMITLI
| 1. | Mentha Varieties – CIM Indus & CIM Madhuras | Commercialized |
| 2. | Bioinformatics softwares: Biosuite & Genocluster | Commercialized |
| 3. | Technology for fractionation of sugarcane bagasse to cellulose, hemicellulose and lignin | Platform technology |
| 4. | Herbal based formulation for diabetes | Licensed |
| 5. | Technology for bio-processing of Leather | Commercialized |
| 6. | A modified catalytic process for economical production of acetic acid, ethylene & vinyl chloride monomer from ethane | Process technology |
| 7. | Diagnostic chips for detecting eye infections and three low vision enhancement devices | Commercialized |
| 8. | Technology for lactic acid production from sugarcane juice by lactobacillus | Platform technology |
| 9. | Technology for production of DHA by microbial fermentation process | Platform technology |
| 10. | Simple office computing (Sofcomp) platform | Commercialized |
| 11. | Darshee – A low cost visualisation platform for bioinformatics | Commercialized |
| 12. | Ashwagandha Varieties – NMITLI 118 and NMITLI 101 | Commercialized |
| 13. | Technology for 3 KWe based on polymer Electrolyte Membrane Fuel Cell - Commissioned at RIL | Commercialized |
| 14. | 500kW horizontal axis wind turbine - Commissioned at Kethanur, TN | Commercialized |
| 15. | Expression vector systems of fungus and yeast for recombinant production of industrially important biomolecules | Platform technology |
| 16. | Technology for Indigenous Dental Implants | Commercialized |
| 17. | Agrotechnology for Jatropha curcas plant for biofuel | Agrotechnology Developed |
| 18. | Triple play broadband technology --adopted by BSNL | Commercialized |
| 19. | Process for production of Tamiflu from shikimic acid | Process technology Developed |
| 20. | Green process for extraction and purification of catechins-Tea polyphenols | Commercialized |
| 21. | Micro PCR device & microchips for diagnosis of TB, Malaria, Dengue, Chikungunya & Hepatitis B | Commercialized |
| 22. | Indigenous Process technology for production of recombinant BNP for diagnosis of congestive heart failure | Process Technology Developed |
| 23. | Technology for 1,2 PDO from Glycerol | Technology Developed |
| 24. | Development of cushion bonded organic ceramic clutch discs | Technology Developed |
| 25. | Novel therapy for management of sepsis | Commercialized |
| 26. | DNA macro-chips based diagnostic test for simultaneous detection of pathogens causing AES (Acute Encephalitic Syndrome) and septicemia | Technology Developed |
| 27. | Intelligent video surveillance solutions | Commercialized |
| 28. | Process for biodiesel production from marine microalgae | Technology developed |
| 29. | ENAMPT – Ultra large naïve human phage display library for therapeutic Mab | Technology Developed |
| 30. | Vaccine against Johnes Disease-JD oil and gel | Commercialized |
| 31. | Novel biomarker (Genes) for detecting head & Neck Cancer | Biomarker signatures discovered |
| 32. | High definition software based video conferencing solution – Vennfer HD | Commercialized |
| 33. | NXR-4D, 4-seat Lithium Ion battery operated electric car | Commercialized |
| 34. | Supercontinnum light source based confocal microscope | Commercialized |
| 35. | Soleckshaw Lite – indigenous electric three wheeler | Commercialized |
| 36. | Nonclonable ID Technology for medical product authentication | Technology Developed |
| 37. | ReaSLR CSM Kit (Reafilter based filtration and concentration of cells by centrifugation) – diagnostic system for testing HIV & TB | Commercialized |
| 38. | Technology for Dimethyl Carbonate from methanol—a nonhazardous process | Technology developed |
| 39. | Computational software “eskin” for predicting effect of chemicals on human skin | Commercialized |
| 40. | Security Ink – Invisible UV sensitive ink | Commercialized |
| 41. | A prototype of high power amplifier (EDFA) designed developed & demonstrated | Under commercialization |
| 42. | Seaweed based formulations for improving health & producing of dairy & poultry animals | Commercialized |
| 43. | Technology for fully automatic ghani plant- commissioned at Khandelia oil mill, Alwar | Commercialized |
| 44. | LT-PEMFC system for automotive application | Technology Demonstrated |
| 45. | 5KW HT PEMFC based cooling & power system for stationary applications | Technology Demonstrated |
NMITLI Projects in Implementation since FY 2020-21:
- Development of Innovative eco-friendly / formaldehyde free Fluorescent Pigments for vast array of water and solvent based applications;
- Development of Novel DPP IV Inhibitor – Phase III Study;
- Developing Dental Implants for Advanced and Critical Applications;
- Development, Manufacturing and Marketing of Micro Raman Spectrometer System with Additional Capabilities of carrying out Photo-Luminescence Spectroscopy and Optical Emission Spectroscopy;
- Development of Novel Antistroke Phyto-pharmaceutical formulation from the roots of a Ashwagandha variety, NMITLI-118; and
- Industrially scalable Ashwagandha (Withania Somnifera) charged formulation for better bone health
NMITLI COVID Projects under Implementation since FY 2020-21:
Considering the crisis due to coronavirus pandemic, CSIR had given a call for proposals from Indian industry under the NMITLI Scheme, especially focusing on coronavirus. CSIR has received 231 proposals by the due date advertised on the CSIR website.
The projects under implementation since FY 2020-21 are:
- Generation of neutralizing human monoclonal antibodies against the SARS-Cov2 virus as a threapeutic strategy to contain the COVID-19 pandemic
- Development of Mycobacterium W for Covid-19: Safety and Efficacy Trial in critically ill, hospitalized and at risk patients
- Development of Ayurveda based botanical drugs for prophylaxis and management of the New Corona Virus Disease (COVID-19)
- Development of an inactivated SARS-CoV2 vaccine for COVID-19 (ICoV2Vac)
- Design and Development of a portable personal Air Purifying Respiratory Device

 Pensioners Corner
Pensioners Corner Screen Reader Access
Screen Reader Access Skip to main content
Skip to main content

























